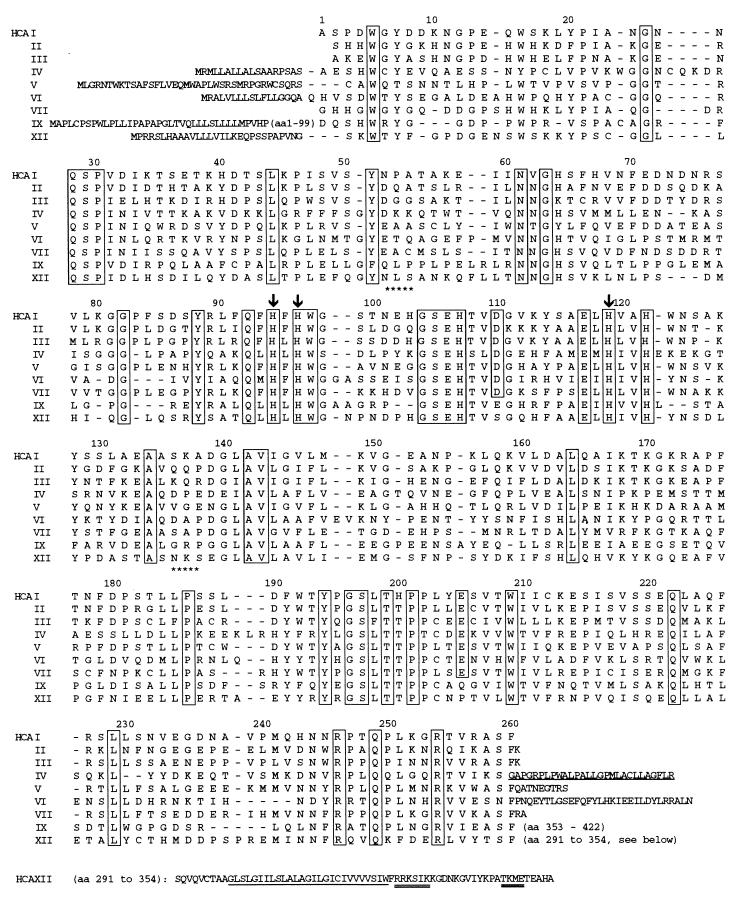
Figure 1.
Amino acid sequences of CA XII aligned below with sequences of other catalytic human CAs. Numbers above the aligned sequences correspond to numbering of amino acids in CA I. The conserved residues are boxed. Arrows above His-94, His-96, and His-119 indicate Zn-binding, active site residues. Stars below NLS and NKS indicate asparagine glycosylation sites. The N-terminal extensions represent the signal sequences of membrane CAs (IV, IX, and XII) and mitochondrial CA V. Cysteines at positions 23 and 203 (CA I numbering) are conserved between CA VI and CA XII. The C-terminal extension of CA XII (amino acid residues 291–354) includes the 26-amino acid hydrophobic domain (underlined) and potential sites for phosphorylation by casein kinase II, protein kinase C, and cAMP-dependent kinase (double underline). The underlined hydrophobic C-terminal extension of CA IV is cleaved off in glycosyl-phosphatidylinositol anchoring and is not found in the mature, glycosyl-phosphatidylinositol-anchored enzyme.