Abstract
The effect of serotonin on membrane potential oscillations of inferior olivary neurones was studied in brainstem slices from 10- to 19-day-old rats.
Serotonin at 50 and 5 μM induced a mean depolarization of 9.4 and 7.7 mV, respectively, that was preceded by a reversible suppression of subthreshold membrane potential oscillations. These effects were not changed by 1 μM tetrodotoxin and the suppression of subthreshold oscillations persisted after current-mediated restoration of resting potential.
In spontaneously active neurones, serotonin abolished the rhythmicity of action potential firing without affecting spike frequency.
Serotonin reduced the slope of the calcium-mediated rebound spike and both the duration and amplitude of the subsequent afterhyperpolarization. Serotonin also shifted the voltage dependence of the rebound spike to more negative values.
Hyperpolarizing current pulses (200 ms) revealed that serotonin increased the pre-rectification and steady-state components of membrane resistance by 37 and 38 %, respectively, in 66 % of neurones, but decreased these parameters by 14 and 20 % in the remaining cells.
The serotonin effects were antagonized by 5 μM methysergide or 1–5 μM ketanserin and were mimicked by 10–20 μM dimethoxy-4-iodoamphetamine but not 10 μM 8-hydroxy-2-(di-N-propylamino)-tetralin.
The data indicate that serotonin suppresses the rhythmic activity of olivary neurones via 5-HT2 receptors by inhibition of the T-type calcium current in combination with membrane depolarization due to activation of a cation current (Ih) and block of a resting K+ current (fast IK(ir)). This modulatory action of serotonin may account for the differential propensity of olivary neurones to fire rhythmically during different behavioural states in vivo.
The inferior olive is a major afferent of the cerebellum and plays an important role in the control of movement. The inferior olive receives substantial innervation from monoaminergic afferents whose effect on olivary activity has not been fully elucidated. Serotonergic inputs are particularly robust from nuclei that sit on the borders of the inferior olive, such as the paragigantocellular reticular nucleus and the raphe obscurus and pallidus (Bishop & Ho, 1986; Compoint & Buisseret-Delmas, 1988). It follows that serotonin plays an important role in motor control by virtue of its conspicuous presence within the inferior olive. Certain disorders of movement of the myoclonic variety have been associated with a loss of brainstem serotonin and signs of cerebellar dysfunction, often without obvious structural damage (Fahn et al. 1986; Welsh et al. 1998).
The membrane properties of olivary neurones provide well-defined sites at which serotonin may act to alter olivary activity. A set of ionic conductances (Llinás & Yarom, 1981a, b, 1986; Yarom & Llinás, 1987; Bal & McCormick, 1997) allows olivary neurones to behave as pacemakers and confer rhythmic potentials to the cerebellum. Specifically, the calcium-dependent potassium conductance, the low-threshold calcium conductance and the hyperpolarization-activated cationic conductance enable olivary neurones to fire rhythmically at 5–12 Hz. Dendritic gap junctions (DeZeeuw, 1990) allow olivary neurones to form electrically coupled groupings. The combination of pacemaker potentials and electrical coupling permits olivary neurones to manifest regular oscillations in membrane potential that are subthreshold for spiking (Llinás & Yarom, 1986; Benardo & Foster, 1986). It has been hypothesized that the coherence of such oscillations provides a means for entraining large aggregates of olivary neurones in order to synchronously activate widespread regions of the cerebellum during movement (Welsh et al. 1995). The role that serotonin plays at the membrane level in modulating olivary rhythmicity remains unknown.
Recent behavioural evidence indicates that the absence of serotonergic fibres in the inferior olive predisposes the motor nervous system towards uncontrollably rhythmic firing. The genetically epilepsy-prone rat (type 3) is a genetically myoclonic rat that shows pathological tremor and rhythmic clonus and which displays a nearly complete loss of serotonergic fibres in its inferior olive (Welsh et al. 1998). That the deficit in olivary serotonin may underlie the disorder in that mutant rat is supported by the finding that ablation of the inferior olive permanently prevented both the tremor and the rhythmic clonus (Welsh et al. 1998). These findings implied that serotonin may have an important role in suppressing the rhythmic output of the inferior olive and that the absence of olivary serotonin may lead to uncontrollably rhythmic movement. This hypothesis was supported by the finding that intraolivary serotonin suppressed rhythmic activity induced by tremorigenic drugs (Headley et al. 1976).
Given the behavioural and neurochemical findings from experiments with the myoclonic rat, we tested the effect of serotonin on olivary electrophysiology in vitro using intracellular recordings. The results unequivocally indicated a powerful role for serotonin in suppressing both ensemble and single-cell oscillations within the inferior olive. Some of the results have been published in abstract form (Placantonakis et al. 1998).
METHODS
The electrophysiology of inferior olivary neurones was studied in brainstem slices of postnatal day (P)10–19 Sprague-Dawley rats. The protocols of Llinás & Yarom (1981a) and Schwarz et al. (1997) were employed with modification. Animal usage was in accordance with local guidelines. Rats were anaesthetized with 15–20 mg ketamine (i.p.) and decapitated. The brain was removed and the brainstem was isolated. A vibratome was used to cut 400–450 μm-thick parasagittal slices in 4°C artificial cerebrospinal fluid (ACSF) which were placed in oxygenated, room temperature ACSF for at least 1–2 h before being moved to a submersion recording chamber where the temperature was increased to 33–35°C over 10 min. ACSF flow of at least 5 ml min−1 perfused the slices in the recording chamber. ACSF consisted of (mM): NaCl 126, KCl 5, NaH2PO4 1.25, MgSO4 2, CaCl2 2, glucose 10 and NaHCO3 26, and was aerated with 95 % O2-5 % CO2 to a final pH of 7.4. ACSF reagents were obtained from Sigma. Slices remained viable for at least 10 h.
Intracellular recordings were obtained using borosilicate glass microelectrodes filled with 3 M potassium acetate with direct current (DC) resistance ranging from 60–100 MΩ. During current clamp, the bridge was frequently checked to ensure adequate series resistance compensation. Only neurones with membrane potentials negative to -55 mV, showing dendritic and somatic calcium conductances, fast sodium spikes and slow anomalous rectification were analysed. Recordings were amplified with an Axoclamp-2B amplifier and acquired with pCLAMP 6 (Axon Instruments) at 10 or 20 kHz. Analysis was performed with pCLAMP 6, Datapac (Run Tech, Laguna Hills, CA, USA), Stranger (Biographics, Chapel Hill, NC, USA) and GB-Stat (Dynamic Microsystems, Silver Springs, MD, USA) software. Curve fitting using the Boltzmann equation was performed with Origin software (Microcal, Northampton, MA, USA). When voltage was differentiated with respect to time, the result was low-pass filtered below 500 Hz. Student's t test and analysis of variance were used with significance accepted for values of P < 0·05; population values are presented as the mean ± 1 s.e.m. Dunnett's test was used for post hoc analysis of the significant F-statistic to allow for all comparisons against a control (Winer, 1971).
The following agents were used: serotonin hydrochloride (RBI, Natick, MA, USA), methysergide (RBI), ketanserin (RBI), dimethoxy-4-iodoamphetamine (DOI; RBI), 8-hydroxy-2-(di-N-propylamino)-tetralin hydrobromide (8-OH-DPAT; RBI), tetrodotoxin (TTX; RBI), ZD7288 (Tocris Cookson, Ballwin, MO, USA) and BaCl2 (Sigma). When BaCl2 was used, MgSO4 was replaced with MgCl2 in the ACSF to ensure solubility of Ba2+. All agents were diluted at least 1000-fold into ACSF from stock solutions.
RESULTS
Intracellular recordings were obtained from 116 inferior olivary neurones, of which 55 showed subthreshold oscillations of membrane potential. The mean resting potential of the neurones was -60.1 ± 0.5 mV. In oscillating neurones, resting potential was calculated from the value half-way between the positive and negative peaks.
Properties of subthreshold oscillations in the rat inferior olive
Subthreshold oscillations of 55 neurones had a mean frequency of 5.1 ± 0.3 Hz (range, 0.5–8.9 Hz) and amplitude of 10.2 ± 1.1 mV (range, 2–22 mV). The oscillations of 14 olivary neurones displayed constant amplitude (Fig. 1A, top), while amplitude modulation patterns were observed in the remaining 41 neurones (Fig. 1A, bottom). Although the frequency range of the subthreshold oscillations was wide (Fig. 1B), each neurone maintained its oscillation frequency in the absence of pharmacological intervention. Depolarization or hyperpolarization of single olivary neurones by DC bias did not abolish or change the frequency of the subthreshold oscillation, although it often altered its form and amplitude (Fig. 1C).
Figure 1. Fundamental properties of rat inferior olivary neurones in vitro.
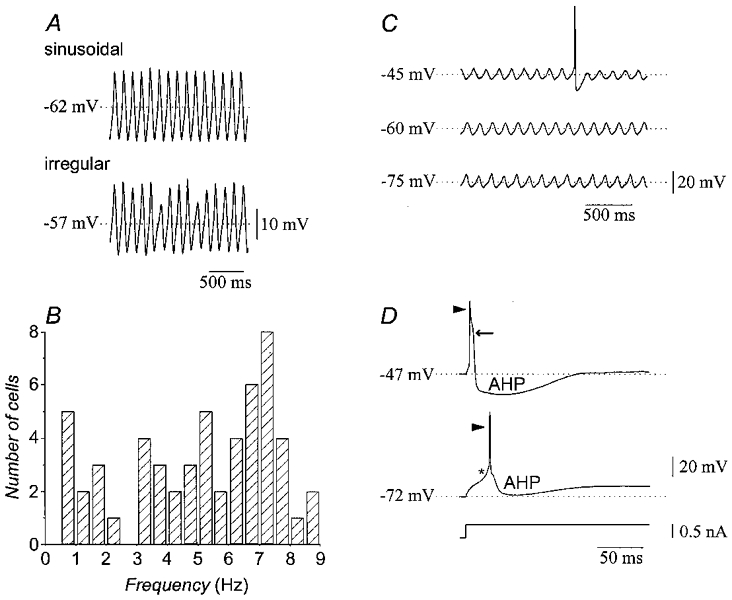
A, recordings from two olivary neurones demonstrating sinusoidal (top) and irregular (bottom) patterns of subthreshold oscillation. B, frequency distribution of subthreshold oscillations in 55 neurones. C, recordings showing the insensitivity of subthreshold oscillations to depolarization (top) and hyperpolarization (bottom). D, stimulation at different membrane potentials reveals two firing modes. At depolarized levels (top), a fast sodium spike (arrowhead) was followed by a high-threshold calcium spike (arrow). At hyperpolarized levels (bottom), a low-threshold calcium spike (asterisk) gave rise to a fast sodium spike (arrowhead). AHP, afterhyperpolarization.
The electrophysiological characteristics of olivary neurones did not change with age. At all ages, depolarizing current pulses elicited action potentials with dendritic calcium components at depolarized holding potentials, while somatic low-threshold calcium spikes became evident at hyperpolarized holding potentials (Fig. 1D), as previously shown (Llinás & Yarom, 1981a). Nevertheless, we found that the probability of recording subthreshold oscillations increased with age (Bleasel & Pettigrew, 1992). Thus, most experiments used rats older than P13 to provide the greatest chance of recording subthreshold oscillations.
Serotonin suppresses subthreshold oscillations but depolarizes olivary neurones and induces spiking
The effects of serotonin, serotonin receptor agonists and serotonin receptor antagonists on the electrophysiological parameters of olivary neurones, including the number of neurones studied and statistical significance, are presented in Table 1. Figure 2A illustrates the two main effects of serotonin. First, at both 50 and 5 μM, serotonin significantly (P < 0·05) and reversibly depolarized olivary neurones. The mean depolarization produced by 50 μM serotonin was 9.4 ± 0.5 mV and for 5 μM serotonin was 7.7 ± 1.0 mV. The difference between the effects of the two concentrations of serotonin was not statistically significant (Fig. 2B). As a consequence of the depolarization, normally quiescent neurones showed spontaneous firing (0.28 ± 0.11 spikes s−1) that could be stopped by hyperpolarizing the membrane with DC bias to the pre-serotonin potential (n = 4). Serotonin at 1 μM did not affect membrane potential or induce spiking (n = 4).
Table 1. Serotonergic agonist and antagonist effects on inferior olivary neurones.
| Agonist/antagonist | Membrane potential (mV) | Oscillation amplitude (mV) | Oscillation frequency (Hz) | Peak slope of rebound (mV ms−1) | Pre-rectification input resistance (MΩ) | Steady-state input resistance (MΩ) | |
|---|---|---|---|---|---|---|---|
| 5-HT (50 μm) | Pre | −60·4 ± 0·6 | Annihilated | Annihilated | 3·0 ± 0·2 | 56·0 ± 4·0 | 44·0 ± 3·5 |
| Post | −51·0 ± 0·6* | oscillations | oscillations | 0·6 ± 0·2*† | 64·5 ± 5·8† | 49·1 ± 3·5† | |
| (48) | (16/16) | (16/16) | (10) | (20) | (16) | ||
| 5-HT (5 μm) | Pre | −59·0 ± 1·1 | Annihilated | Annihilated | 3·0 ± 0·2 | 56·0 ± 4·0 | 44·0 ± 3·5 |
| Post | −51·3 ± 1·4* | oscillations | oscillations | 0·6 ± 0·2*† | 64·5 ± 5·8† | 49·1 ± 3·5† | |
| (20) | (12/14) | (12/14) | (10) | (20) | (16) | ||
| 5-HT (1 μm) | Pre | −58·3 ± 1·8 | 11·5 ± 4·3 | 6·9 ± 0·3 | n. d. | n. d. | n. d. |
| Post | −58·0 ± 1·8 | 11·6 ± 4·3 | 6·8 ± 0·4 | n. d. | n. d. | n. d. | |
| (4) | (4) | (4) | |||||
| 5-HT + methysergide (5 μm)† | Pre | −60·4 ± 1·3 | 16·5 ± 3·5 | 6·4 ± 0·7 | 2·9 ± 0·2 | 45·6 ± 16·4 | n. d. |
| Post | −59·9 ± 1·3 | 16·4 ± 3·8 | 6·7 ± 0·4 | 2·8 ± 0·1 | 47·1 ± 17·6 | n. d. | |
| (11) | (4) | (4) | (3) | (3) | |||
| 5-HT (5 μm) + ketanserin (1 μm) | Pre | −59·0 ± 1·8 | 10·1 ± 1·8 | 5·4 ± 1·8 | 3·6 ± 0·4 | 48·7 ± 9·8 | 36·9 ± 5·9 |
| Post | −59·3 ± 2·0 | 9·5 ± 1·3 | 5·3 ± 1·8 | 3·6 ± 0·5 | 46·7 ± 7·8 | 31·4 ± 4·9 | |
| (9) | (6) | (6) | (3) | (4) | (4) | ||
| DOI (10—20 μm) | Pre | −60·7 ± 0·6 | Annihilated | Annihilated | 3·1 ± 0·4 | 40·9 ± 17·8 | 32·8 ± 17·4 |
| Post | −51·0 ± 1·6* | oscillations | oscillations | 0·5 ± 0·2* | 69·9 ± 34·2 | 64·6 ± 34·2 | |
| (8) | (3/3) | (3/3) | (3/5) | (4) | (4) | ||
| 8-OH-DPAT (10 μm) | Pre | −58·5 ± 1·7 | 8·3 ± 2·8 | 6·2 ± 0·4 | 2·6 ± 0·3 | 57·9 ± 10·8 | 49·9 ± 7·9 |
| Post | −59·3 ± 1·9 | 8·9 ± 3·5 | 6·1 ± 0·5 | 2·4 ± 0·2 | 56·6 ± 11·4 | 50·6 ± 9·5 | |
| (13) | (4) | (4) | (3) | (8) | (8) |
P < 0.05, as compared to Pre.
Data pooled for 50 and 5 μm 5HT. Numbers in parentheses are the number of neurones studied. n. d., not determined.
Figure 2. Effects of serotonin on subthreshold oscillations and mean membrane potential.
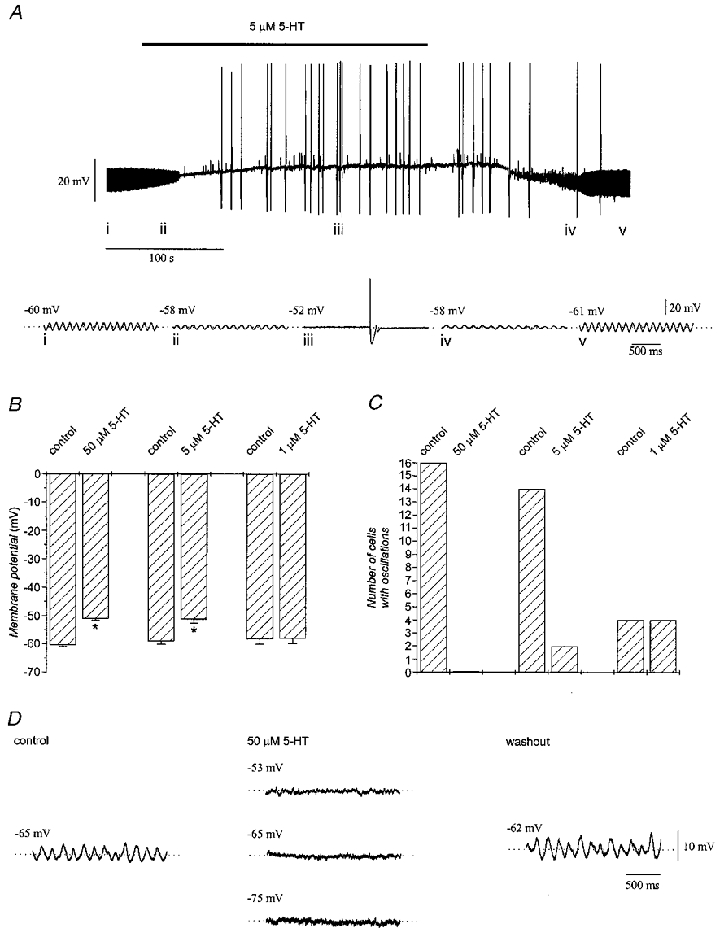
A, recording from an olivary neurone showing the reversible suppression of subthreshold oscillations, membrane depolarization and increased excitability with 5 μM serotonin (top). Faster sweep-speed traces illustrate the effect in greater detail (i-v, bottom). B, mean membrane potential before and during application of 50 μM (n = 48), 5 μM (n = 20) and 1 μM (n = 4) serotonin. Asterisks indicate significant change from control (P < 0·05). C, number of inferior olivary neurones showing subthreshold oscillations before and during application of 50 μM (n = 16), 5 μM (n = 14) and 1 μM (n = 4) serotonin. D, abolition of subthreshold oscillations by serotonin could not be reversed when the membrane potential was restored to pre-serotonin levels or further hyperpolarized by DC bias.
The second main effect of serotonin was to completely and reversibly suppress the subthreshold oscillations of membrane potential in 28 out of 30 neurones tested with 50 or 5 μM serotonin (Fig. 2C). At 50 μM, serotonin abolished the subthreshold oscillations in all neurones tested while 5 μM serotonin had the same effect in 86 % of neurones tested. In the two neurones in which subthreshold oscillations were not abolished by 5 μM serotonin, there was nevertheless a reduction in the peak-to-peak amplitude of the oscillations from 9.3 mV before serotonin to 5.5 mV in the presence of serotonin, without a change in oscillation frequency (8.0 and 7.9 Hz before and in the presence of serotonin, respectively), suggesting a graded effect on oscillation amplitude but not frequency. In addition, oscillations could be suppressed repeatedly in the same neurone within an interval as short as 10 min after washout (n = 4). Serotonin at 1 μM affected neither the amplitude nor the frequency of subthreshold oscillations (Table 1).
The suppression of the oscillations with serotonin could not be reversed by restoring the membrane potential of the recorded neurone with sustained current injection to its pre-serotonin value or by further polarization (n = 4; Fig. 2D). This finding indicated that the effect of serotonin on subthreshold oscillations was not simply a consequence of the change in membrane potential of the recorded neurone. The suppression of oscillations and membrane depolarization produced by 50 μM serotonin persisted in the presence of 1 μM TTX (n = 4; Fig. 3). This finding suggested that serotonin acted directly on the olivary neurones.
Figure 3. Abolition of subthreshold oscillations by serotonin is mediated postsynaptically.
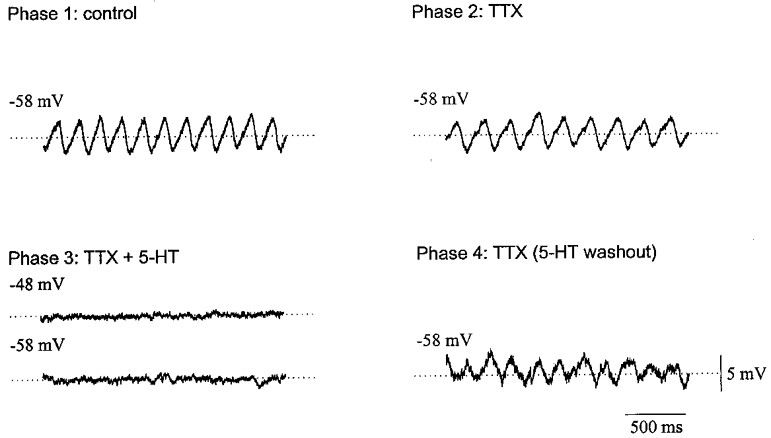
Under control conditions (Phase 1), the olivary neurone represented here had a membrane potential of -58 mV and showed 6 Hz subthreshold oscillations. In Phase 2, addition of 1 μM TTX did not significantly affect the resting potential or the oscillation. In Phase 3, application 50 μM serotonin and 1 μM TTX abolished the oscillations and depolarized the membrane to -48 mV. Returning the membrane potential to -58 mV did not restore the oscillation. In Phase 4, serotonin was removed from the bath and the oscillations returned.
The abolition of subthreshold oscillations by serotonin is independent of changes in population membrane potential
Subthreshold oscillations of the inferior olive require a globally active low-threshold calcium current (IT) between neurones throughout the nucleus (Lampl & Yarom, 1997). The fact that serotonin suppressed subthreshold oscillations while depolarizing the membrane raised the possibility that the loss of the oscillation was a simple consequence of inactivating the low-threshold calcium conductance due to global depolarization. Two observations argued against this possibility.
The first and simplest observation was that serotonin often abolished subthreshold oscillations before it depolarized the neurone (Fig. 4A), indicating a primary action on the conductances underlying the oscillations independent of the effect on conductances responsible for the depolarization. The second observation was made in four experiments in which the membrane potential of the entire neuronal population was manipulated by reducing the concentration of K+ in the perfusate prior to applying serotonin (Fig. 4B). Reducing the K+ concentration from 5 mM to 1.5 mM hyperpolarized olivary neurones by 6–8 mV and abolished subthreshold oscillations (compare Fig. 4B i with B ii), presumably due to global deactivation of the low-threshold calcium conductance (Lampl & Yarom, 1997). When 50 μM serotonin was added to the bath containing 1.5 mM K+, the membrane was depolarized to control levels (Fig. 4B iii), but oscillations were not restored. Subsequent removal of serotonin from the low K+ perfusate reversed the depolarization (Fig. 4B iv) and, afterwards, the oscillations were quickly restored by bringing the K+ concentration back to 5 mM (Fig. 4B v). These findings demonstrated that serotonin exerted actions on the subthreshold oscillations that were independent of its actions on changing the membrane potential of the neuronal population within the inferior olive.
Figure 4. Independence of the action of serotonin on mean membrane potential and subthreshold oscillations.
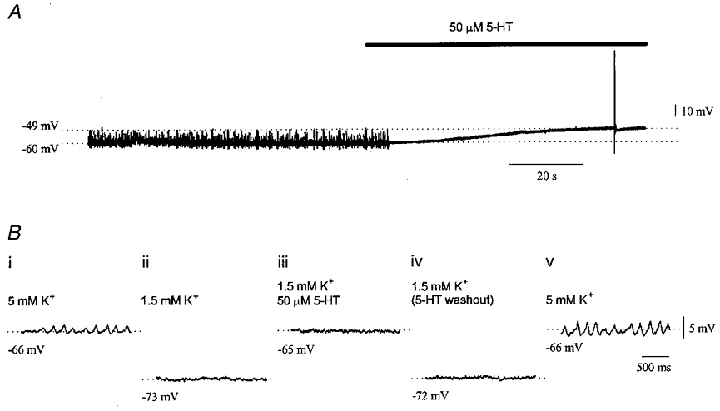
A, recording from an olivary neurone demonstrating that 50 μM serotonin suppressed subthreshold oscillations before it depolarized the membrane. B, a 5-phase experiment manipulating K+ concentration showing the independence of the abolition of subthreshold oscillations by serotonin from membrane potential. In phase 1 (i) resting potential was -66 mV and the neurone showed 6 Hz subthreshold oscillations in the presence of 5 mM K+. In phase 2 (ii), 1.5 mM K+ hyperpolarized the neurone to -73 mV and abolished the oscillations. In phase 3 (iii), 50 μM serotonin depolarized the neurone to the control level but could not restore the subthreshold oscillations. In phase 4 (iv), serotonin was removed and the membrane potential returned to -72 mV and remained quiescent. Returning the K+ concentration to 5 mM in phase 5 (v) restored both the membrane potential and the oscillations.
Olivary serotonin suppresses spike rhythmicity
Five neurones exhibited spontaneous spiking under control conditions. Autocorrelation of the spiking indicated that it was rhythmic (P < 0·05) in all five cases and had a frequency of 7.1 ± 0.4 Hz. The rhythmic spiking was phase-locked to subthreshold oscillations of the membrane potential in four out of five cases. In all cases, 50 μM serotonin abolished the rhythmicity of the spiking in addition to depolarizing the neurone and abolishing the subthreshold oscillations (Fig. 5). Such changes occurred without a change in spike frequency (0.23 ± 0.04 and 0.29 ± 0.10 spikes s−1 before and in the presence of serotonin, respectively; P > 0·05).
Figure 5. Progression of the abolition of subthreshold and suprathreshold rhythmicity by serotonin.
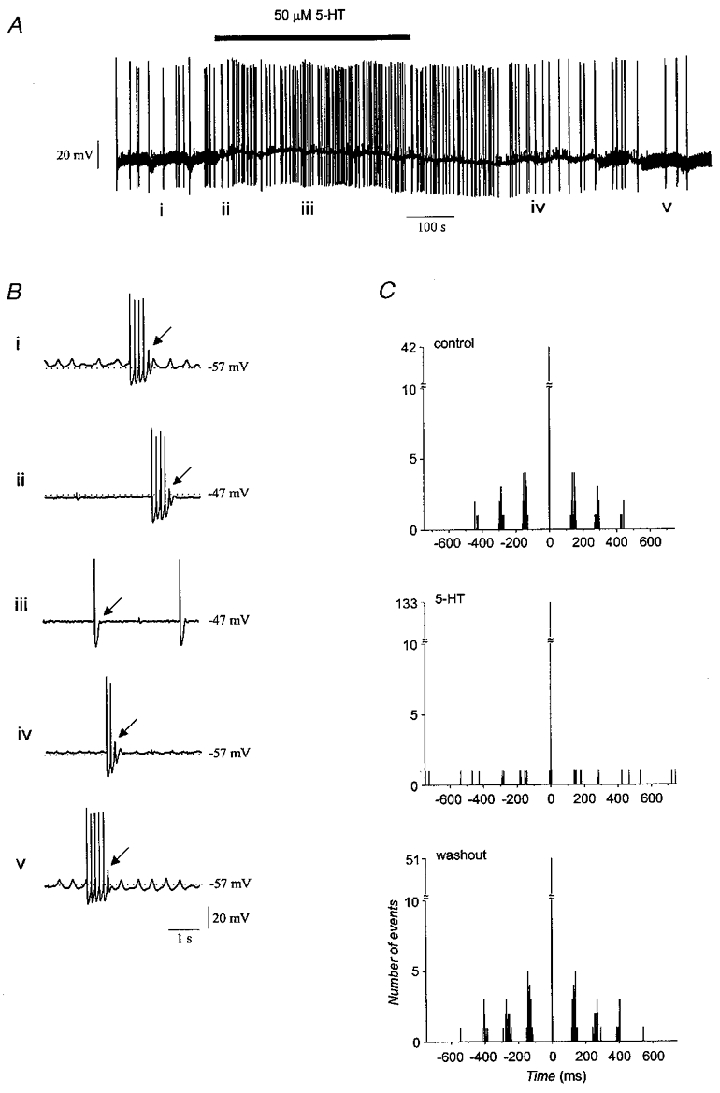
A, a 20 min recording showing that serotonin blocked subthreshold oscillations and depolarized the neurone. B, faster sweep-speed traces showing representative activity at different times during the experiment. Under control conditions (i), bursts of rhythmic spikes with a frequency of 7 Hz were initiated on the rising edge of a 2–3 Hz subthreshold oscillation. Application of serotonin initially blocked the subthreshold oscillation without affecting the occurrence or frequency of spike bursts (ii). Shortly thereafter, spike bursts were abolished and spiking was arrhythmic (iii). Removing serotonin allowed rhythmic bursts to return (iv) followed later by recovery of the subthreshold oscillation (v). Arrows indicate rebounds that failed to elicit sodium spikes. C, autocorrelograms of spiking before and in the presence of serotonin, and after its removal. Bin width is 2 ms.
Analysis of spontaneously spiking neurones that simultaneously manifested subthreshold oscillations showed that serotonin induced an orderly progression from rhythmic to arrhythmic activity (Fig. 5B). The first effect of serotonin was to abolish the subthreshold oscillations without affecting the rhythmicity of spiking within multiple spike bursts (Fig. 5B ii). Approximately 10 s later, serotonin blocked spike rhythmicity by reducing rebound potentials. Subsequently, within a few minutes, rebound potentials were completely abolished (Fig. 5B iii). The reverse progression was observed when serotonin was removed from the perfusate (Fig. 5B iv and Bv). It appeared that repetitive spiking was more resistant to disruption by serotonin than was the subthreshold oscillation. This was probably due to the greater depth of the hyperpolarization produced within spike bursts, which could provide a more effective recruitment of low-threshold calcium conductances.
Serotonin inhibits conductances that underlie rhythmic activity
The effect of serotonin on the ability of olivary neurones to fire rhythmically in response to hyperpolarizing current pulses was determined. The analysis was restricted to neurones that had a stable membrane potential so as to remove the facilitative effect of subthreshold oscillations on rhythmic spiking and to allow precise measurements of input resistance and rectification. We assumed, like Bal & McCormick (1997), that the properties of neurones with stable membrane potential were not different from those of oscillating neurones.
Under control conditions, terminating a 200 ms hyperpolarizing current pulse evoked a train of action potentials (Fig. 6Ai) that ranged in frequency from 6 to 11 Hz (n = 10). As shown by Bal & McCormick (1997), such trains of action potentials are triggered by the low-threshold calcium spike upon release from hyperpolarization which, in turn, triggers a sodium spike. During application of 50 μM (n = 6) or 5 μM (n = 4) serotonin, the repetitive spiking triggered by release from hyperpolarization was abolished both at the depolarized and at the control potential re-established by DC bias (Fig. 6Aii). The effect was reversed a few minutes after removal of serotonin (Fig. 6Aiii).
Figure 6. Effect of serotonin on repetitive spiking and spike parameters.
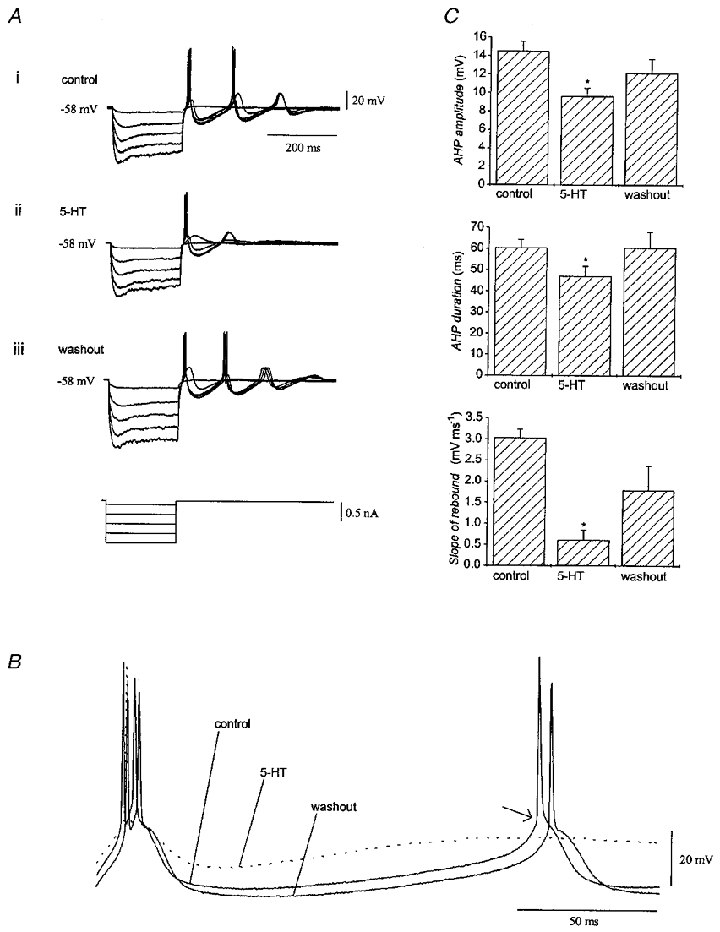
A, recordings from an olivary neurone showing that repetitive spiking elicited by terminating hyperpolarizing current pulses (i) was reversibly suppressed by 50 μM serotonin (ii). The membrane potential was corrected with DC bias while serotonin was in the bath. B, fast sweep-speed traces from a different neurone immediately following release from hyperpolarization before (control), during and after application of serotonin to show its effect on repetitive spiking. Note that serotonin decreased the amplitude of the afterhyperpolarization and blocked the rebound potential that led to the second spike in the train. The arrow indicates the inflection point in the potential used for discriminating the rebound calcium spike from the sodium spike. C, parameters of spikes elicited by release from hyperpolarization before, in the presence of, and after removal of serotonin. Data were pooled from experiments using 50 and 5 μM serotonin (n = 10). Asterisks indicate P < 0·05.
Mean spike parameters and their alteration by serotonin were calculated from the spike trains triggered by release from hyperpolarization at the pre-serotonin holding potential (n = 10). Only events elicited from deep hyperpolarization were used for comparisons to ensure that a full-blown initial calcium spike was elicited under all conditions. The slope of the calcium rebound was derived by differentiating membrane potential with respect to time from the 30 ms before the second action potential, which had an onset that could be clearly distinguished by the inflection point in the potential trace (Fig. 6B, arrow). The analysis (Fig. 6C) indicated that 50 or 5 μM serotonin: (a) reduced the peak amplitude of the first afterhyperpolarization from 14.5 ± 1.1 to 9.6 ± 0.9 mV (P < 0·05); (b) reduced the duration of the afterhyperpolarization at its mid-amplitude (given by half the difference between peak hyperpolarization and rest) from 60.4 ± 3.9 to 47.0 ± 4.8 ms (P < 0·05); and (c) reduced the peak slope of the second rebound potential from 3.0 ± 0.2 to 0.6 ± 0.2 mV ms−1 (P < 0·05; Table 1). The reduction of the peak slope of the rebound was independent of the effect of serotonin on input resistance, as determined by regression (r = 0·35, P = 0·41).
Similar experiments were repeated in the presence of 1 μM TTX, thereby allowing the first rebound potential triggered by the release from hyperpolarization to be analysed in isolation. Figure 7A shows the firing of a neurone after release from hyperpolarization under normal conditions. Here, the stimulus elicited a low-threshold calcium spike (left-hand filled arrow) which, in turn, triggered an action potential (open arrow). Serotonin at 50 μM (Fig. 7B) prevented the action potential, apparently by decreasing the slope of the initial low-threshold spike, without altering input resistance. Pretreatment of two neurones with TTX (Fig. 7C) unmasked the low-threshold calcium spike triggered by the stimulus. Adding 50 μM serotonin to the TTX-treated neurones decreased the peak slope of the response elicited by release from hyperpolarization while increasing the steady-state input resistance (Fig. 7D), indicating that serotonin inhibited the low-threshold spike directly.
Figure 7. Effect of serotonin on the low-threshold calcium spike.
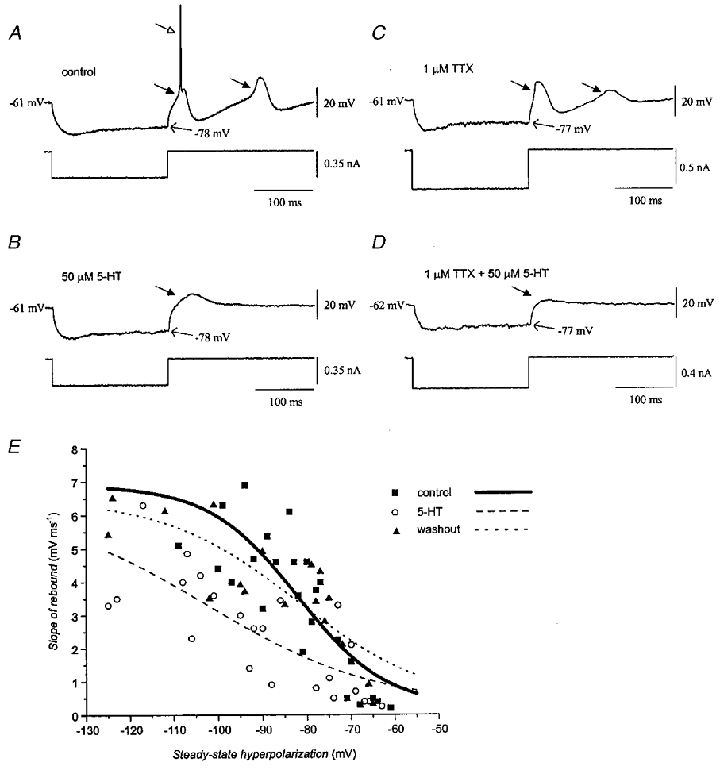
A, control record illustrating that release from hyperpolarization elicited a low-threshold calcium spike (filled arrow), in turn triggering a sodium spike (open arrow). In this trial, the rebound low-threshold spike that followed the afterhyperpolarization did not trigger a sodium spike. B, 50 μM serotonin prevented the elicited low-threshold spike from triggering a sodium spike or a following rebound. Same neurone as in A. C, in another neurone, TTX unmasked the low-threshold calcium spike and the ensuing calcium rebound. D, adding 50 μM serotonin to the TTX-treated neurone in C suppressed the low-threshold calcium spike. E, plots of the slope of the low-threshold spike triggered by release from hyperpolarization before (▪, continuous line), during (^, dashed line), and after (▴, dotted line) application of serotonin. Data from seven neurones were plotted as a function of the steady-state level of hyperpolarization produced by different current injections and were pooled from neurones that received 50 μM (n = 4) and 5 μM (n = 3) serotonin. Curves were calculated with the Boltzmann equation y = (A1–A2)/(1 + exp((V–V½)/g)) +A2, where A1 and A2 are the minimum and maximum values of the slope of the rebound, V½ is the membrane potential at which the slope of the rebound potential reached half-maximum, g is the slope factor, and y and V represent the slope of the rebound and the membrane potential, respectively. V½ was -82, -102 and -82 mV; g was -10, -19 and -15 mV; A1 was 0.2, 0.3 and 0.3 mV ms−1; and A2 was 6.9, 6.3 and 6.5 mV ms−1 for the control, serotonin and washout conditions, respectively.
To quantify the effect of serotonin on the low-threshold conductance, we plotted data from seven neurones describing the slope of the rebound potentials elicited upon release from various depths of hyperpolarization in the absence of TTX. The curves in Fig. 7E were calculated using the Boltzmann equation and were derived from the pooled data of the seven neurones. The analysis showed a sigmoidal relationship between the steady-state depth of hyperpolarization and the slope of the elicited rebound potential before serotonin (Fig. 7E, continuous curve), proposed by Llinás & Yarom (1986) to represent the voltage dependence of inactivation of the low-threshold calcium channels. Serotonin (Fig. 7E, dashed curve) reduced the slope factor of this function by 9 mV and shifted it to the left by 20 mV. The voltage sensitivity of the low-threshold conductance was nearly restored 10–15 min after serotonin was removed (Fig. 7E, dotted curve). Analysis of variance of the entire set of raw data binned in 20 mV blocks indicated a significant effect of drug (F2,64= 37.6; P < 0·01) and post hoc tests indicated that the slope of the rebound spike during serotonin application (t2,70= 3.0; P < 0·01), but not after its removal (t2,70= 0.04; P > 0·05), was significantly different from the pre-serotonin value. The effect was observed in all seven neurones studied. There was not a significant correlation between the change in the peak slope of the rebound potential and a change in input resistance (r = 0.7, P = 0·12). Similar findings were obtained from two neurones treated with TTX, in which serotonin reduced the slope factor by 2 mV and shifted it to the left by 4 mV.
Mechanisms of serotonin-induced depolarization: effects on fast potassium inward rectifier current (IK(ir)) and Ih
Experiments were performed to determine the currents upon which serotonin acted to depolarize olivary neurones. First, we determined the effect of serotonin on the input resistance of 21 neurones, using 200 or 400 ms hyperpolarizing current pulses (0.3–0.6 nA). As previously demonstrated (Yarom & Llinás, 1987), olivary neurones show a biphasic voltage response to hyperpolarization, the so-called anomalous rectification, with slow inward rectification activated a few tens of milliseconds following current injection. The pre-rectification response is mediated primarily by IK(ir), while the latter rectification is mediated by the non-specific cation and hyperpolarization-activated current Ih (Yarom & Llinás, 1987; Bal & McCormick, 1997). The input resistance of olivary neurones prior to rectification was 56.0 ± 4.0 MΩ, while the resistance was 44.0 ± 3.5 MΩ at steady state. The effect of serotonin on input resistance varied among neurones, but was not a function of drug concentration. The majority (66 %) of neurones showed an increase in input resistance with serotonin, while the remainder showed a decrease. For the former group, serotonin increased the pre-rectification and steady-state input resistance by 37 ± 11 and 38 ± 12 %, respectively. For the latter group, serotonin decreased pre-rectification and steady-state input resistance by 14 ± 4 and 20 ± 6 %, respectively. Figure 8 shows two examples of the effect of serotonin on input resistance. In one neurone (Fig. 8A), 5 μM serotonin increased the pre-rectification and steady-state input resistance by 34 and 47 %, respectively. In the other neurone (Fig. 8B), 50 μM serotonin decreased the pre-rectification and steady-state input resistance by 34 and 35 %, respectively. The distribution of changes among the 21 neurones is shown in Fig. 8C.
Figure 8. Effects of serotonin on input resistance.
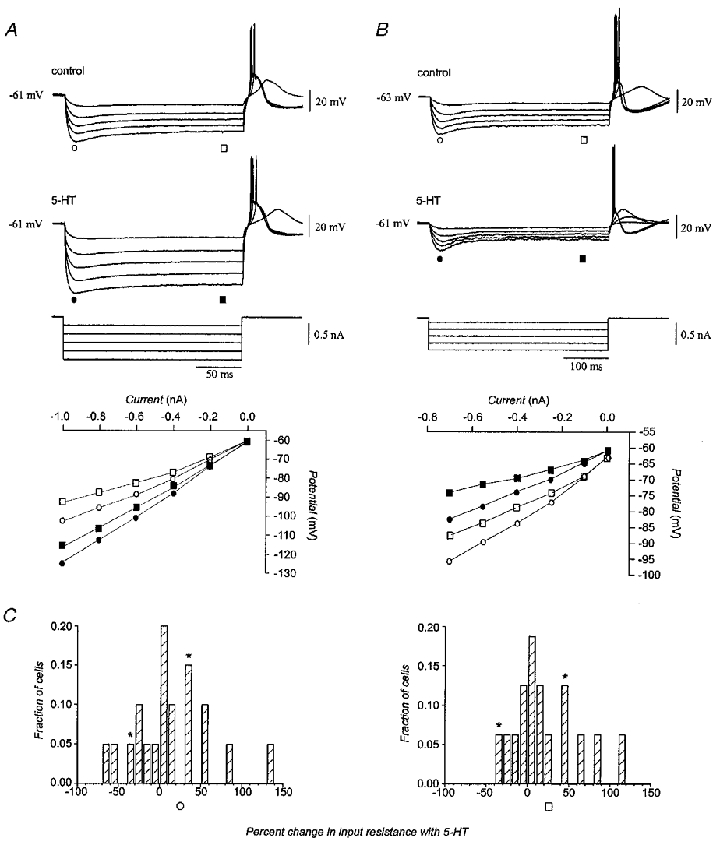
A, recordings from a neurone in which serotonin increased pre-rectification and steady-state input resistance by 34 and 47 %, respectively. B, recordings from another neurone in which serotonin decreased pre-rectification and steady-state input resistance by 34 and 35 %, respectively. Plots of current-voltage relations are presented for the pre-rectification (circles) and steady-state (squares) stages of the voltage response before (open symbols) and in the presence of (filled symbols) serotonin. During application of serotonin the membrane potential was corrected with DC bias to the pre-serotonin level. C, distribution of changes in input resistance with serotonin measured at peak hyperpolarization (n = 20; left) and at steady state (n = 16; right). Asterisks indicate the neurones shown in A and B.
The possibility that a modulation of IK(ir) contributed to the depolarization produced by serotonin was studied using extracellular Ba2+, a blocker of this current (Hagiwara et al. 1978; Yarom & Llinás, 1987). Application of 0.5 mM Ba2+ to olivary neurones increased pre-rectification input resistance by 45 % (25.3 ± 2.3 to 36.9 ± 3.7 MΩ; n = 3; P < 0·05; Fig. 9A and B) and depolarized the membrane by 12 mV (-63.1 ± 1.6 to -50.5 ± 2.0 mV; n = 13; P < 0·05), consistent with a blockade of IK(ir) (Yarom & Llinás, 1987). After re-establishing the control membrane potential with DC bias, Ba2+ significantly attenuated the ability of 50 μM serotonin to depolarize the membrane by 38 % (n = 4; P < 0·05; Fig. 9C). Ba2+ also blocked the ability of serotonin to increase input resistance in three out of three neurones tested and, moreover, unmasked a tendency for serotonin to decrease input resistance. Under such conditions, 50 μM serotonin decreased both the pre-rectification and steady-state input resistance by 12 % (31.1 ± 4.2 to 27.7 ± 4.9 MΩ; P < 0·05) and 6 % (24.4 ± 6.3 to 23.1 ± 6.1 MΩ; P = 0·22), respectively (Fig. 9D). The experiment indicated that the increase in input resistance could be accounted for by a primary action to inhibit IK(ir), but also that another current was facilitated by serotonin.
Figure 9. Effect of serotonin on fast inward rectification.
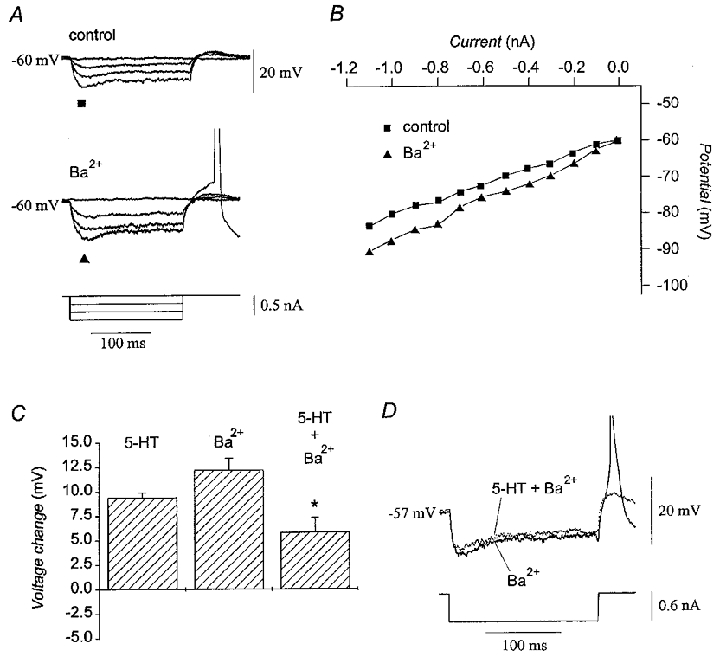
A, treatment of a representative neurone with 0.5 mM Ba2+ attenuated fast inward rectification of the membrane potential. B, current-voltage plot of the peak hyperpolarizion before (▪) and after (▴) the addition of 0.5 mM Ba2+ for the neurone in A. For both A and B, the original membrane potential was restored with DC bias to the control level while Ba2+ was present. C, magnitude of the depolarization produced by 50 μM serotonin (n = 48), by 0.5 mM Ba2+ (n = 13), and by the combination of 50 μM serotonin and 0.5 mM Ba2+ (n = 4). After correction of the membrane potential with DC bias, serotonin depolarized the membrane by 5.8 ± 1.5 mV in the presence of Ba2+ as compared to a depolarization of 9.4 ± 0.5 mV when 50 μM serotonin was applied alone. Asterisk indicates P < 0·05 compared to serotonin alone. D, membrane potential traces in response to a -0.6 nA pulse in the presence of 0.5 mM Ba2+ alone (continuous line) and during application of 50 μM serotonin and 0.5 mM Ba2+ (dotted line) showing that serotonin decreased input resistance in the presence of Ba2+. Action potentials have been truncated.
Analysis of the neurones showing decreased input resistance indicated an action of serotonin to facilitate the hyperpolarization-activated cationic current (Ih). In 5 out of 16 neurones, serotonin decreased input resistance at both the pre-rectification and steady-state time points (Fig. 10A) and enhanced slow inward rectification as a function of peak hyperpolarization (Fig. 10B and C). To determine whether an enhancement of Ih may have underlain the depolarization of olivary neurones by serotonin, the effect of the specific Ih blocker ZD7288 (BoSmith et al. 1993) on the modulation of membrane potential by serotonin was tested. Addition of 20 μM ZD7288 (n = 4) hyperpolarized the membrane by 5 mV (-60.8 ± 0.4 to -65.3 ± 1.0 mV; P < 0·05), eliminated the slow component of inward rectification during hyperpolarizing current pulses (Fig. 10D), and increased both components of input resistance by 31 % (57.3 ± 12.2 to 72.7 ± 13.6 MΩ; P < 0·05) and 75 % (45.0 ± 12.3 to 71.4 ± 13.3 MΩ; P < 0·05), respectively (Fig. 10E). More to the point, ZD7288 significantly attenuated the ability of 50 μM serotonin to depolarize the membrane by 38 % (n = 4; P < 0·05; Fig. 10F). The findings indicated that the serotonin-induced depolarization of olivary neurones was mediated partially by enhancing Ih, in addition to inhibiting IK(ir). Similar findings have been reported in other neuronal types (North & Uchimura, 1989; Larkman & Kelly, 1998).
Figure 10. Effect of serotonin on Ih.
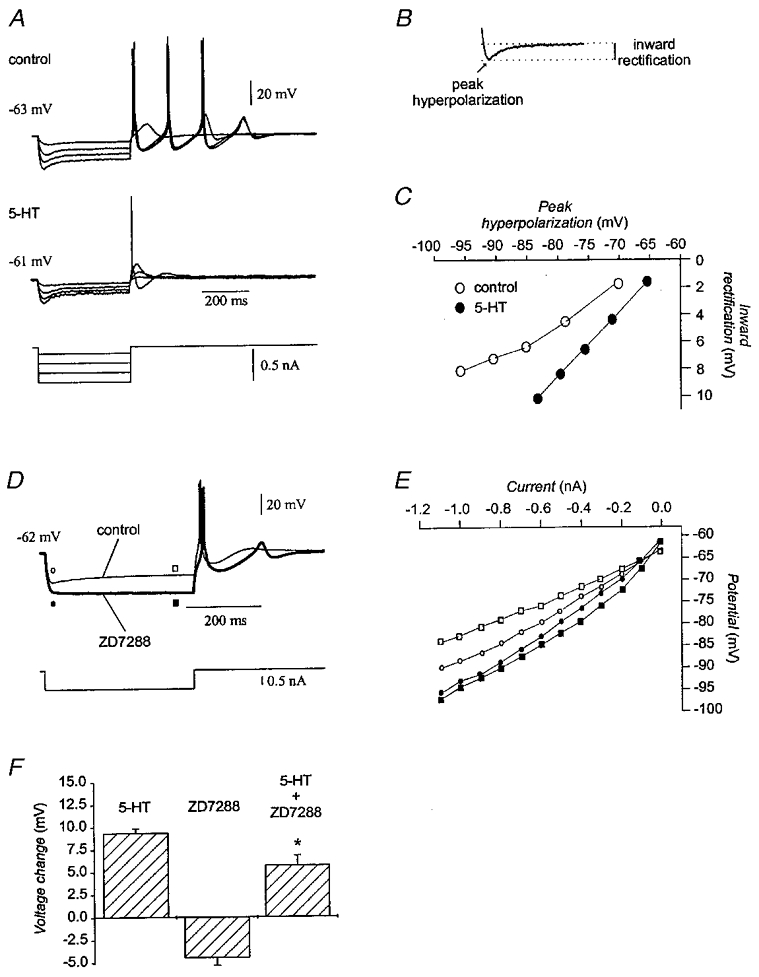
A, recordings from the neurone shown in Fig. 8B in which serotonin decreased input resistance. The membrane potential was restored to the pre-serotonin resting value with DC bias during application of serotonin. B, inward rectification was defined as the difference between the peak hyperpolarization and the steady-state potential. C, plot of the magnitude of inward rectification as a function of peak hyperpolarization before (^) and in the presence of 50 μM serotonin (•) for the neurone in A. D, recordings from an olivary neurone to which 20 μM ZD7288 was added. The membrane potential was restored with DC bias to the control value during application of ZD7288. Ih contributes to input resistance prior to the slow inward rectification, because the current is partly active at rest. E, current-voltage plot constructed from the recordings in D showing that ZD7288 (filled symbols) increased both the pre-rectification (circles) and steady-state (squares) input resistance as compared to the control (open symbols). F, magnitude of the depolarization produced by 50 μM serotonin (n = 48), 20 μM ZD7288 (n = 4), and 50 μM serotonin and 20 μM ZD7288 together (n = 4). After correcting the potential with DC bias, serotonin depolarized the membrane by 5.8 ± 1.1 mV in the presence of ZD7288 as compared to a depolarization of 9.4 ± 0.5 mV with 50 μM serotonin alone. Asterisk indicates P < 0·05.
Withdrawing serotonin induces subthreshold oscillations
Another phenomenon of interest was observed in five neurones that had a stable, non-oscillating membrane potential prior to serotonin application. As with all other olivary neurones, serotonin depolarized the membrane and induced spiking (Fig. 11A). However, withdrawing serotonin not only allowed these neurones to repolarize but also induced subthreshold oscillations within a few minutes (Fig. 11B). The subthreshold oscillations ranged from 3 to 7 Hz and persisted through the remainder of the experiment, which lasted for up to 1 h. Autocorrelation of the subthreshold activity before and after addition of serotonin indicated that withdrawing serotonin induced a rhythmic state in membrane potential that was not present prior to its application (Fig. 11C). The induction of rhythmicity by applying and then removing serotonin from olivary neurones was indicative of desensitization of serotonin receptors resulting in withdrawal-like symptoms.
Figure 11. Withdrawing serotonin from olivary neurones induces subthreshold oscillations.
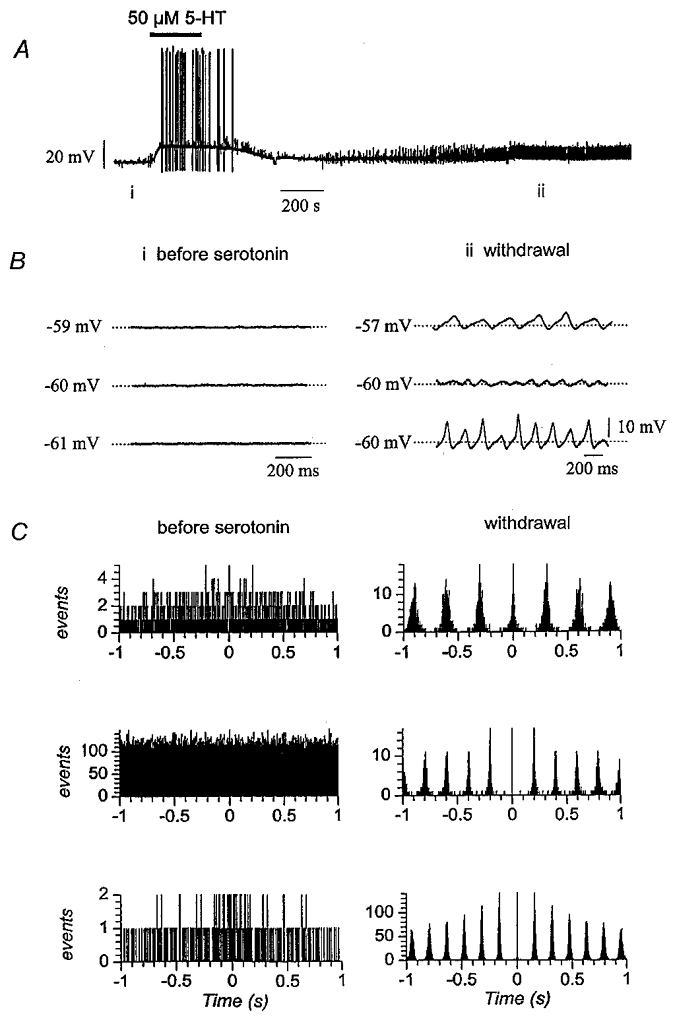
A, recording from an olivary neurone in which subthreshold oscillations were induced by withdrawing serotonin. B, faster sweep-speed traces prior to serotonin (i) and after withdrawing serotonin (ii) from three neurones. C, autocorrelation histograms of the membrane potential from the three neurones shown in B prior to applying serotonin and after withdrawal. Events were defined as moments in which the membrane potential exceeded its mean plus 1.6 standard deviations. Bin width is 1 ms.
Serotonin exerts its effects on inferior olivary neurones via 5-HT2 receptors
All of the effects of 50 and 5 μM serotonin were blocked by pretreatment with 5 μM methysergide, a non-specific serotonin receptor antagonist (Table 1). In the presence of methysergide, serotonin did not significantly depolarize the neurones (n = 11) or change the amplitude or the frequency of subthreshold oscillations (n = 4). Moreover, serotonin did not alter the input resistance or the slope of the rebound during application of methysergide (n = 3; all P > 0·05). Methysergide by itself had no consistent effect on the electrophysiology of the neurones.
Ketanserin, a specific antagonist of the 5-HT2 receptor (Van Nueten et al. 1981), blocked all effects of serotonin on olivary neurones (Table 1). In the presence of 1 μM ketanserin, 5 μM serotonin did not depolarize the neurones (n = 9) and did not alter the amplitude or the frequency of subthreshold oscillations (n = 6; Fig. 12A and B). In addition, 1 μM ketanserin antagonized the effect of 5 μM serotonin on input resistance (n = 4) and the slope of the rebound potential (n = 3; all P > 0·05; Fig. 12C and D). In two neurones, pretreatment with 5 μM ketanserin completely blocked the effect of 50 μM serotonin on subthreshold oscillations. Like methysergide, ketanserin by itself did not consistently alter the electrophysiology of olivary neurones.
Figure 12. Antagonism of the effects of serotonin by ketanserin.
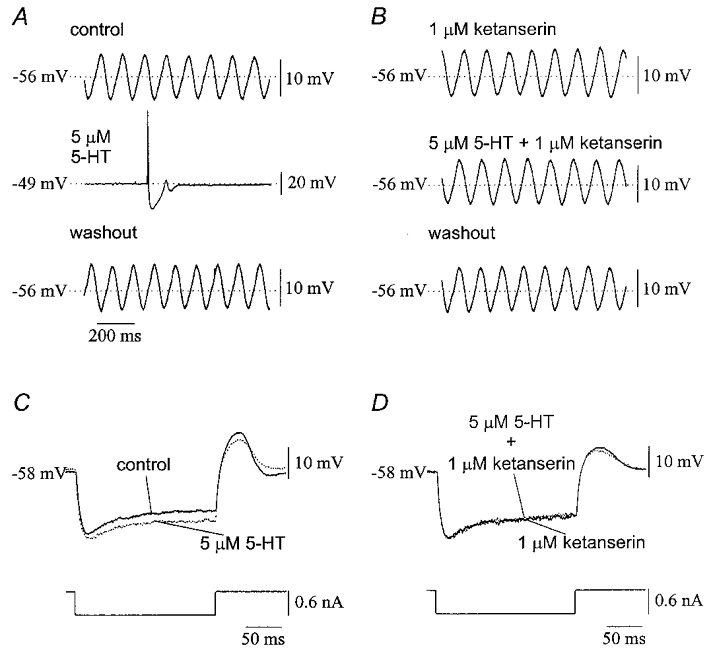
A, application of 5 μM serotonin to an olivary neurone reversibly depolarized the membrane by 7 mV and blocked subthreshold oscillations in membrane potential. B, application of 1 μM ketanserin to the same neurone as in A did not affect the membrane potential or the subthreshold oscillation but completely blocked the effect of 5 μM serotonin. C, traces from a neurone under control conditions (continuous line) and in the presence of 5 μM serotonin (dotted line) showing the increase in input resistance and reduction of the rebound potential with serotonin. D, the effects were antagonized by 1 μM ketanserin (same neurone as in C).
The specific 5-HT2 agonist DOI (Shannon et al. 1984) mimicked all of the effects of serotonin at concentrations of 10–20 μM (Table 1). DOI suppressed subthreshold oscillations in three neurones (Fig. 13A), depolarized the membrane by 9.7 ± 1.6 mV (n = 8), and reduced the peak slope of rebound potentials in three of five neurones (Fig. 13B). Moreover, DOI significantly enhanced the pre-rectification and steady-state components of input resistance by 66.8 ± 17.5 and 99.0 ± 15.0 % (n = 4; P < 0·05, calculated from a t test of difference from 0 % change), respectively (Fig. 13C). In three of five neurones with stable membrane potential, DOI briefly induced oscillations during the slow depolarization. Overall, DOI reproduced all of the effects observed with serotonin itself, except for the decrease in input resistance, which was not observed in the four neurones tested.
Figure 13. Effects of DOI and 8-OH-DPAT on olivary neurones.
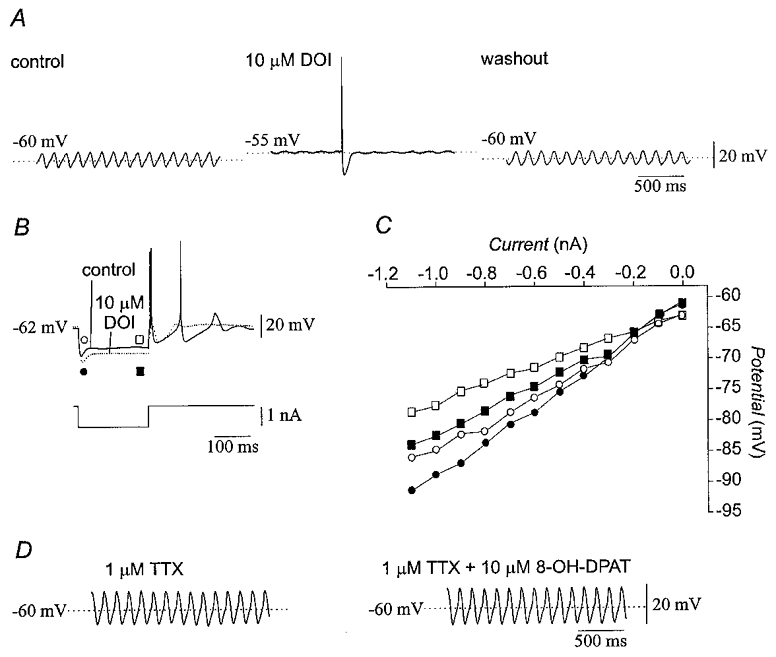
A, 10 μM DOI reversibly suppressed subthreshold oscillations and depolarized the membrane of olivary neurones. B, recordings from a representative olivary neurone showing that 10 μM DOI increased input resistance and suppressed repetitive spiking (dotted line). The original membrane potential was restored with DC bias during application of DOI. C, current-voltage plot constructed from the experiment shown in B indicating that DOI (filled symbols) increased the pre-rectification (circles) and steady-state (squares) input resistances as compared to the control condition (open symbols). D, treatment with 10 μM 8-OH-DPAT in the presence of 1 μM TTX did not change subthreshold oscillations or membrane potential.
In contrast, 10 μM 8-OH-DPAT, a specific agonist of the 5-HT1A receptor (Middlemiss & Fozard, 1983), did not affect the amplitude or the frequency of subthreshold oscillations in the presence of 1 μM TTX (n = 4; Fig. 13D), the peak slope of rebound potentials (n = 3), membrane potential (n = 13), or input resistance (n = 8; all P > 0·05; Table 1). These findings indicated that 5-HT2 receptors mediated the effects of serotonin on olivary neurones, except possibly for the decrease in input resistance for which antagonist and agonist data were not obtained.
DISCUSSION
The experiments in this study demonstrated that serotonin is a very powerful suppressor of the rhythmic activity of the inferior olive. Serotonin blocked both subthreshold oscillations and the rhythmicity of action potentials of inferior olivary neurones. Serotonin inhibited the low-threshold T-type calcium current, IT, and the currents that underlie afterhyperpolarization. These actions formed a common basis for the effect of serotonin on both subthreshold and suprathreshold rhythmicity within the inferior olive. Moreover, serotonin powerfully depolarized olivary neurones as a result of its combined ability to inhibit the fast potassium inward rectifier current, IK(ir), and to facilitate the hyperpolarization-activated cation current, Ih. However, the fact that the ability of serotonin to modulate input resistance was not correlated with its ability to change the slope of the rebound potential indicated that its actions on IK(ir) and Ih were distinct from its actions on IT. Together, the specific action of serotonin on the conductances underlying rhythmicity and general actions on the resting potential provide a potent combination of mechanisms to reduce the rhythmicity of inferior olive neurones.
The actions of serotonin upon olivary neurones were mediated by the 5-HT2 receptor. The main effects of serotonin were blocked by ketanserin and replicated by DOI, antagonist and agonist of 5-HT2 receptors, respectively. The modulation by serotonin of olivary conductances was consistent with an activation of the second messenger pathways that are activated by 5-HT2 receptors. It has been established that 5-HT2 receptors are coupled to Gq proteins, which transduce the binding of serotonin into activation of the phospholipase C-protein kinase C second messenger system (Saudou & Hen, 1994). Activation of protein kinase C with phorbol esters inhibited the low-threshold calcium conductance in dorsal root ganglia (Schroeder et al. 1990) and the calcium-activated potassium conductance in Aplysia neurones (Critz & Byrne, 1992). Moreover, serotonin suppressed the low-threshold calcium conductance in Xenopus neurones (Sun & Dale, 1997) and reduced the calcium-dependent potassium conductance in trigeminal motoneurones (Inoue et al. 1999). Thus, the 5-HT2 receptor-mediated effects on olivary neurones were consistent with effects that have been observed previously.
The experiments showed that serotonin depolarized olivary neurones by acting on two currents, Ih and IK(ir), in turn, producing bidirectional changes in input resistance in different neurones. Although unitary effects were observed in single neurones, the direction of modulation of each current by serotonin was consistent with depolarization of the membrane: IK(ir), which has an equilibrium potential more negative than rest, was inhibited, while Ih, which has a reversal potential at -40 mV (Pape, 1996), was enhanced. Yet, the majority of olivary neurones studied showed an increase in both instantaneous and steady-state input resistance in the presence of serotonin, indicating a predominant effect of serotonin to inhibit IK(ir), which could have masked a less prominent effect on Ih. Nevertheless, the importance of the modulation of Ih by serotonin was reinforced by the 33 % of neurones that showed an enhancement of the slow inward rectification. However, neither DOI nor 8-OH-DPAT replicated the ability of serotonin to decrease input resistance and enhance slow inward rectification, leaving open the possibility that receptors other than the 2 and 1A subtype mediated the modulation of Ih by serotonin.
The results indicated that serotonin acts concertedly at multiple subcellular elements to suppress the rhythmic activity of olivary neurones. First, by acting on the soma, serotonin suppressed the low-threshold calcium conductance that is responsible for both the depolarizing phase of the subthreshold oscillation and the rebound spike that enables repetitive spiking; this is a direct effect on the primary rhythm-generating element of olivary neurones. Other direct effects of serotonin also acted to suppress rhythmic activity. A second action was to enhance Ih and inhibit IK(ir) in order to depolarize the neurone and inactivate the low-threshold calcium conductance. This secured the action of serotonin at the soma to inhibit IT. One consequence of depolarization was to bring the membrane potential closer to the threshold for spiking, thereby increasing excitability. A third action has been suggested by the finding in pyramidal cells that the density of channels mediating Ih is greatest in higher-order dendrites (Magee, 1998). Within the inferior olive, higher-order dendrites are the sites where spines form electrical synapses with neighbouring neurones. If Ih is greatest at higher-order dendrites of olivary neurones, serotonin would increase the number of open channels in the dendrites and shunt current flowing from coupled spines to the soma (Bleasel & Pettigrew, 1994).
The combined ability of serotonin to inhibit the low-threshold calcium conductance and to depolarize olivary neurones indicates that serotonin engages redundant mechanisms for blocking olivary rhythmicity that are adapted to the anatomy of the serotonergic system. Serotonergic input to the inferior olive is non-synaptic (King et al. 1984), as it is in other regions (e.g. Descarries et al. 1991), suggesting a paracrine influence mediated by volume transmission (Fuxe & Agnati, 1991). The majority of release sites on serotonergic fibres within the olive are apposed to higher-order dendrites lacking synaptic specialization, while a small percentage of fibres surround somata (King et al. 1984). The volume of the typical olivary dendritic arbor and the packing density of olivary somata indicate that each arbor encompasses more than 100 somata (DeZeeuw, 1990), creating a network that allows all types of subcellular elements to be in close proximity to serotonergic terminals. A recent study determined that the paracrine effects of serotonin could extend up to 20 μm from each release site and last for up to 200 ms before uptake and metabolic mechanisms removed it from the extracellular space (Bunin & Wightman, 1998), suggesting that all components of olivary neurones can be influenced by serotonin.
The present results are contrasted by contradictory results from in vivo experiments. The first studies showed that serotonin attenuated olivary tremor (Bowman & Osuide, 1968; Goldstein et al. 1969), that inhibition of serotonin synthesis exacerbated olivary tremor (Kelley & Naylor, 1974), and that serotonin delivered into the olive suppressed the rhythmicity of spiking (Headley & Lodge, 1976; Headley et al. 1976). The finding of these experiments – suppression of olivary rhythmicity – agrees with that of the present study. Yet, the opposite conclusion was reached by Sugihara et al. (1995), who showed in rats that intraolivary serotonin induced rhythmic firing. One possible explanation for the result of Sugihara et al. (1995) is that the effects of serotonin seen in that study may have been due to receptor desensitization and not the immediate effect of serotonin itself. This explanation is supported by the fact that the rhythmic firing recorded by Sugihara et al. (1995) persisted for up to 1 h, an outcome inconsistent with the multiple reuptake and metabolic mechanisms that rapidly remove serotonin from brain (Bunin & Wightman, 1998) but analogous to the finding that application and then withdrawal of serotonin from quiescent olivary neurones induced sustained oscillations (Fig. 11). However, developmental factors cannot be ruled out entirely, given the different ages of the animals employed.
What is the role of olivary serotonin? Our data indicate that serotonin may act as a ‘switch’ for olivary neurones to induce a state transition in their temporal characteristics of firing and their responsiveness to synaptic input. The experiments demonstrated that serotonin causes the transition of olivary neurones through three states. The first state occurs when serotonin is absent and is characterized by subthreshold oscillations in membrane potential that can entrain rhythmic spiking. The second state occurs at the early stage of serotonin application and is characterized by rhythmic spiking in the absence of subthreshold oscillations. The third state occurs during optimal levels of serotonin, when the neurones are depolarized, subthreshold oscillations are lost and spiking is arrhythmic.
The ability of serotonin to regulate olivary rhythmicity may have important implications for the function of serotonin in movement. Serotonergic input to the inferior olive originates mainly from the medullary raphe (Bishop & Ho, 1986; Compoint & Buisseret-Delmas, 1988). Such neurones are nearly silent during desynchronized sleep, more active during slow-wave sleep and quiet wakefulness, and most active during movement (Veasey et al. 1995). The state dependence of the firing of the serotonergic input to the inferior olive predicts that olivary rhythmicity should be weakest during skilled movement and greatest during desynchronized sleep. Olivary neurones are rhythmic when animals are anaesthetized (Bell & Kawasaki, 1972; Sasaki et al. 1989) or quietly awake (Lang et al. 1999), and become hyperrhythmic during the transition from slow-wave to desynchronized sleep (Marchesi & Strata, 1970; Hobson & McCarley, 1972). The rhythmicity manifested by olivary neurones in these states was consistent with low serotonergic tone. In contrast, rhythmicity of single olivary neurones has been difficult to observe during skilled movement (Keating & Thach, 1995), consistent with high serotonergic tone (Veasey et al. 1995). It is important to note that this characterization does not diminish the significance of rhythmicity in movement control, because the activity summed across ensembles of olivary neurones may be rhythmic and timed to specific movement parameters (Welsh et al. 1995).
A consequence of the actions of serotonin may be that discrete releases of serotonin might subdivide groups of synchronously oscillating olivary neurones into subgroups, thereby allowing them to act independently of one another. Moreover, serotonin may provide functional versatility to olivary neurones by switching them back and forth from a resting state, in which they behave rhythmically due to the intrinsic characteristics of their membranes, to a state in which they are continuously close to the threshold for responding to synaptic input. In this role, serotonin may integrate olivary neurones into a synaptic circuitry in order to permit sensory feedback or motor throughput to influence movement via the olivocerebellar system.
Finally, it is noteworthy that an impairment of the brainstem serotonin system has been implicated in certain forms of myoclonus – a disorder often manifested as highly rhythmic and synchronous muscle jerks. It is well known that drugs which increase serotonergic neurotransmission can be effective anti-myoclonic therapies (Fahn et al. 1986). A recent study showed a virtual absence of serotonergic fibres in the inferior olive of a mutant rat that exhibited profound tremor and clonus (Welsh et al. 1998). On these grounds, it was hypothesized that a loss of olivary serotonin predisposes the motor system to uncontrolled oscillation and synchrony. The hypothesis received strong support from the finding that removal of the inferior olive reversed that rat's myoclonic syndrome (Welsh et al. 1998). The present study reinforces the view that a lack of serotonin may predispose olivary neurones to rhythmic activity – a state which may contribute to certain forms of rhythmic myoclonus.
Acknowledgments
This research was supported by United States Public Health Service Grant NS31224 from the United States National Institute for Neurological Disorders and Stroke, The Myoclonus Research Foundation, and grant DFG SCHW577/4 (C.S.). The authors thank Dr J. A. Harvey for helpful discussions.
References
- Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current Ih. Journal of Neurophysiology. 1997;77:3145–3156. doi: 10.1152/jn.1997.77.6.3145. [DOI] [PubMed] [Google Scholar]
- Bell CC, Kawasaki T. Relations among climbing fiber responses of nearby Purkinje cells. Journal of Neurophysiology. 1972;35:155–169. doi: 10.1152/jn.1972.35.2.155. [DOI] [PubMed] [Google Scholar]
- Benardo L, Foster E. Oscillatory behavior in inferior olive neurons: mechanism, modulation, cell aggregates. Brain Research Bulletin. 1986;17:773–784. doi: 10.1016/0361-9230(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH. Cell bodies of origin of serotonin-immunoreactive afferents to the inferior olivary complex of the rat. Brain Research. 1986;399:369–373. doi: 10.1016/0006-8993(86)91530-1. [DOI] [PubMed] [Google Scholar]
- Bleasel A, Pettigrew A. The effect of bicarbonate-free artificial cerebrospinal fluid on spontaneous oscillations of the membrane potential in inferior olivary neurons of the rat. Brain Research. 1994;639:8–20. doi: 10.1016/0006-8993(94)91758-2. [DOI] [PubMed] [Google Scholar]
- Bleasel AF, Pettigrew AG. Development and properties of spontaneous oscillations of the membrane potential in inferior olivary neurons in the rat. Brain Research, Developmental Brain Research. 1992;65:43–50. doi: 10.1016/0165-3806(92)90006-i. [DOI] [PubMed] [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea pig dissociated sinoatrial node cells. British Journal of Pharmacology. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WC, Osuide G. Interaction between the effects of tremorine and harmine and of other drugs in chicks. European Journal of Pharmacology. 1968;3:106–111. doi: 10.1016/0014-2999(68)90060-5. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: An investigation of extrasynaptic transmission. Journal of Neuroscience. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compoint C, Buisseret-Delmas C. Origin, distribution and organization of the serotonergic innervation in the inferior olivary complex of the rat. Archives of Italian Biology. 1988;126:99–110. [PubMed] [Google Scholar]
- Critz SD, Byrne JH. Modulation of IK, Ca by phorbol ester-mediated activation of PKC in pleural sensory neurons of Aplysia. Journal of Neurophysiology. 1992;68:1079–1086. doi: 10.1152/jn.1992.68.4.1079. [DOI] [PubMed] [Google Scholar]
- Descarries L, Séguéla P, Watkins KC. Nonjunctional relationships of monoamine axon terminals in the cerebral cortex of adult rat. In: Fuxe K, Agnati LF, editors. Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission. New York: Raven Press; 1991. pp. 53–62. [Google Scholar]
- DeZeeuw CI. Ultrastructure of the Cat Inferior Olive. Rotterdam, The Netherlands: Universiteits Erasmus Drukkerij; 1990. [Google Scholar]
- Fahn S, Marsden CD, Van Woert MH. Advances in Neurology. vol. 43. New York: Raven Press; 1986. Myoclonus. [PubMed] [Google Scholar]
- Fuxe K, Agnati LF. Two principal modes of electrochemical communication in the brain: Volume versus wiring transmission. In: Fuxe K, Agnati LF, editors. Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission. New York: Raven Press; 1991. pp. 1–9. [Google Scholar]
- Goldstein M, Battista AF, Nakatani S, Angagnoste B. The effects of centrally acting drugs on tremor in monkeys with mesencephalic lesions. Proceedings of the National Academy of Sciences of the USA. 1969;63:1113–1116. doi: 10.1073/pnas.63.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Moody W, Patalak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. The Journal of Physiology. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley PM, Lodge D. Studies on field potentials and on single cells in the inferior olivary complex of the rat. Brain Research. 1976;101:445–459. doi: 10.1016/0006-8993(76)90470-4. [DOI] [PubMed] [Google Scholar]
- Headley PM, Lodge D, Duggan AW. Drug-induced rhythmical activity in the inferior olivary complex of the rat. Brain Research. 1976;101:461–478. doi: 10.1016/0006-8993(76)90471-6. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW. Spontaneous discharge rates of cat cerebellar Purkinje cells in sleep and waking. Electroencephalography and Clinical Neurophysiology. 1972;33:457–469. doi: 10.1016/0013-4694(72)90210-6. [DOI] [PubMed] [Google Scholar]
- Inoue T, Itoh S, Kobayashi M, Kang Y, Matsuo R, Wakisaka S, Morimoto T. Serotonergic modulation of the hyperpolarizing spike afterpotential in rat jaw-closing motorneurons by PKA and PKC. Journal of Neurophysiology. 1999;82:626–637. doi: 10.1152/jn.1999.82.2.626. [DOI] [PubMed] [Google Scholar]
- Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in awake behaving monkey is random. Journal of Neurophysiology. 1995;73:1329–1340. doi: 10.1152/jn.1995.73.4.1329. [DOI] [PubMed] [Google Scholar]
- Kelley DM, Naylor RJ. Mechanisms of tremor induction by harmine. European Journal of Pharmacology. 1974;27:14–24. doi: 10.1016/0014-2999(74)90197-6. [DOI] [PubMed] [Google Scholar]
- King JS, Ho RH, Burry RW. The distribution and synaptic organization of serotoninergic elements in the inferior olivary complex of the opossum. Journal of Comparative Neurology. 1984;227:357–368. doi: 10.1002/cne.902270306. [DOI] [PubMed] [Google Scholar]
- Lampl I, Yarom Y. Subthreshold oscillations and resonant behavior: two manifestations of the same mechanism. Neuroscience. 1997;78:325–341. doi: 10.1016/s0306-4522(96)00588-x. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Welsh JP, Llinás R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. Journal of Neuroscience. 1999;19:2728–2739. doi: 10.1523/JNEUROSCI.19-07-02728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Characterization of 5-HT-sensitive potassium conductances in neonatal rat facial motoneurones in vitro. The Journal of Physiology. 1998;508:67–81. doi: 10.1111/j.1469-7793.1998.067br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. The Journal of Physiology. 1981a;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. The Journal of Physiology. 1981b;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. The Journal of Physiology. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi GF, Strata P. Climbing fibers of cat cerebellum: modulation of activity during sleep. Brain Research. 1970;17:145–148. doi: 10.1016/0006-8993(70)90317-3. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-N-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. European Journal of Pharmacology. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- North RA, Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. The Journal of Physiology. 1989;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:200–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Placantonakis DG, Schwarz C, Welsh JP. Serotonin suppresses subthreshold oscillations in the inferior olive. Society for Neuroscience Abstracts. 1998;24:665. [Google Scholar]
- Sasaki K, Bower JM, Llinás R. Multiple Purkinje cell recording in rodent cerebellar cortex. European Journal of Neuroscience. 1989;1:572–586. doi: 10.1111/j.1460-9568.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Saudou F, Hen R. 5-Hydroxytryptamine receptor subtypes: molecular and functional diversity. Advances in Pharmacology. 1994;30:327–380. doi: 10.1016/s1054-3589(08)60178-7. [DOI] [PubMed] [Google Scholar]
- Schroeder J, Fischbach PS, McCleskey EW. T-type calcium channels: Heterogeneous expression in rat sensory neurons and selective modulation by phorbol esters. Journal of Neuroscience. 1990;10:947–951. doi: 10.1523/JNEUROSCI.10-03-00947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Möck M, Thier P. Electrophysiological properties of rat pontine nuclei neurons in vitro. I. Membrane potentials and firing patterns. Journal of Neurophysiology. 1997;78:3323–3337. doi: 10.1152/jn.1997.78.6.3323. [DOI] [PubMed] [Google Scholar]
- Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA) European Journal of Pharmacology. 1984;102:23–29. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: A multiple-electrode study in the rat cerebellum. European Journal of Neuroscience. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Sun Q-Q, Dale N. Serotonergic inhibition of the T-type and high voltage-activated Ca2+ currents in the primary sensory neurons of Xenopus larvae. Journal of Neuroscience. 1997;17:6839–6849. doi: 10.1523/JNEUROSCI.17-18-06839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nueten JM, Janssen PA, Van Beck J, Xhonneux R, Verbeuren TJ, Vanhoutte PM. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. Journal of Pharmacology and Experimental Therapeutics. 1981;218:217–230. [PubMed] [Google Scholar]
- Veasey SD, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. Journal of Neuroscience. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Chang B, Menaker ME, Aicher SA. Removal of the inferior olive abolishes myoclonic seizures associated with a loss of olivary serotonin. Neuroscience. 1998;82:879–897. doi: 10.1016/s0306-4522(97)00297-2. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Sugihara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- Yarom Y, Llinás R. Long-term modifiability of anomalous and delayed rectification in guinea pig inferior olivary neurons. Journal of Neuroscience. 1987;7:1166–1177. doi: 10.1523/JNEUROSCI.07-04-01166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


