Figure 2. Responses to FMRFamide of intact oocytes, and outside-out patches taken from oocytes, injected with HtFaNaC cRNA.
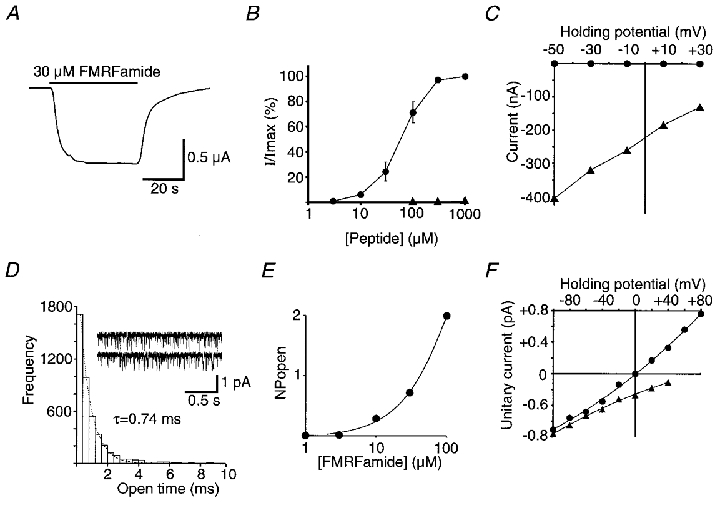
A, example of a response showing little desensitization to the applied FMRFamide. B, dose-response relationship of HtFaNaC to FMRFamide (•). Also shown are data from responses obtained from one oocyte with FLRFamide (▴). I/Imax is the amplitude of the response at a given concentration divided by the maximum response to FMRFamide. The points for FMRFamide represent the mean of responses from six experiments with different oocytes. The EC50 value for FMRFamide is approximately 70 μM. Error bars correspond to the s.e.m.C, relationship between the holding potential and the amplitude of the FMRFamide response of HtFaNaC recorded in the normal oocyte physiological solution (▴), and in physiological solution with all the Na+ replaced with K+ (•). All the points in this graph were obtained from one oocyte and demonstrate a very much higher permeability of the channel to Na+ than to K+. D, the open time frequency distribution of the unitary currents activated by 10 μM FMRFamide recorded from an outside-out patch of an oocyte that had been injected with HtFaNaC cRNA. The data were fitted with a single exponential distribution (dotted line), with τ = 0.74 ms. Example traces from the same patch are also shown. The patch potential was -100 mV. In this and all subsequent unitary current recordings, the inward currents are shown as downward deflections from the baseline using the standard convention. E, an incomplete dose-response curve showing the relationship between FMRFamide concentration and level of channel activity in a patch assessed by the summed products of the number of channels open in the patch and the probability of that number of channels being open (NPopen). The patch contained at least four channels. The holding potential was -100 mV. F, the dependency of the amplitude of the unitary currents on the holding potential of the membrane patch. In normal extracellular physiological solution and with CsF in the recording pipette (▴), the currents were inward in sign at +40 mV, suggesting a preferential permeability of the channel to Na+. With the recording electrode filled with NaF to give the same concentration of Na+ across the patch (•), the currents reversed at 0 mV, confirming selective permeability to Na+.
