Figure 2. Hypothetical mechanism of calcium-dependent gating in BKCa channels.
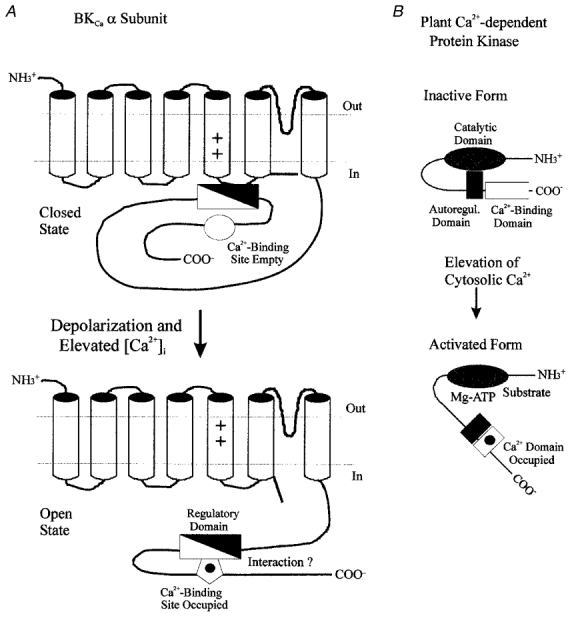
The calcium-induced leftward shift of voltage-dependent gating in BKCa channels (A) may occur by a mechanism similar to that described for the activation of Ca2+-dependent protein kinases by calcium-CaM (Kemp & Pearson, 1991; Ito et al. 1991; Knighton et al. 1992; Nairn & Picciotto, 1994). In the latter situation, an intrinsic autoregulatory domain interacts with the enzyme's catalytic site, keeping it inactive. In the plant Ca2+-dependent protein kinase (B), this inactivation is removed by calcium binding to a CaM-like domain contained within the enzyme's primary structure, thereby allowing substrate and ATP to access the catalytic site. In BKCa channels, an analogous regulatory segment (filled triangle) may also exist, that could, for example, constrain movement of the channel's voltage sensor. Binding of calcium to its intrinsic site may then allow interaction of this site with a region of the regulatory segment (open triangle), and thus remove the constraint on the channel's gating mechanism. The intrinsic calcium-binding site of the BKCa channel may thus act in a similar fashion to Ca2+-CaM in the activation of Ca2+-dependent protein kinases.
