Figure 4.
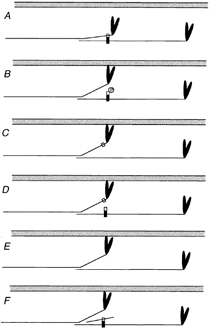
Unifying model for the impact of C-protein modifications on crossbridge behaviour
Each panel shows a schematic representation of a thin filament (grey) and a pair of myosin molecules from a thick filament that are axially staggered appropriately to be crosslinked by a C-protein molecule (vertical rectangle). The C-terminal part of the C-protein (black) binds to the light meromyosin part of the tail, the N-terminal part (white) to S2. C-protein is too short (∼40 nm) to span the distance (> 50 nm) between the binding sites in the S2 and light meromyosin portions of a single myosin molecule, therefore it crosslinks molecules. A shows the quiescent state, in which we propose that S2 is tethered by C-protein. In B, phosphorylation of C-protein releases the S2 part (Weisberg & Winegrad, 1996), allowing the myosin heads greater ease of interaction with the thin filament, thereby promoting thin filament activation. C, C-protein, truncated at the C-terminus (Yang et al. 1998) cannot crosslink. D, exogenous C1C2 domains compete with endogenous C-protein, releasing the S2 part, but do not compete if phosphorylated (Kunst et al. 2000). E, extraction of C-protein (Hofmann et al. 1991) removes the tether. F, exogenous S2 can compete with the S2 part of myosin (this work), releasing the tether. C–F thus all mimic B. The model predicts that weakening of the interactions between myosin and C-protein caused by mutations in the S2 part of myosin heavy chain will have effects similar to these other interventions.
