Figure 1. Consequences of mitochondrial Ca2+ uptake for mitochondrial function.
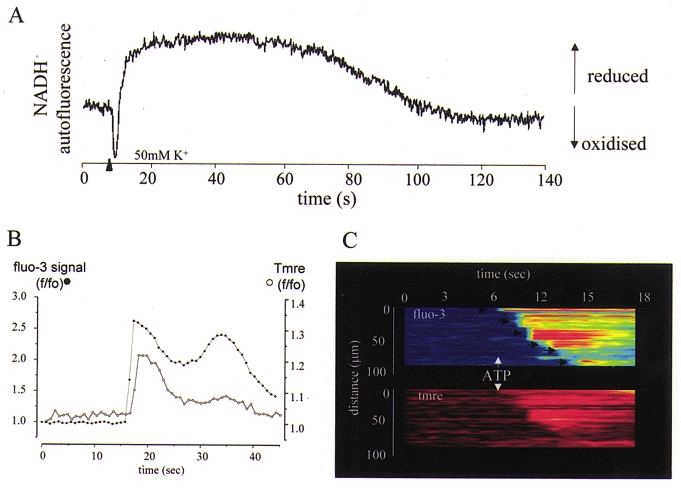
A, changes in NADH autofluorescence, excited at 350 nm and recorded at 450 nm, are shown following a 100 ms pulse of 50 mm KCl, which was used to depolarise a mouse sensory neuron and thence to raise [Ca2+]c. The autofluorescence initially showed a transient decrease attributable to a transient depolarisation of ΔΨm that accompanies the Ca2+ flux into mitochondria (see below and Fig. 4). This was then superseded by a prolonged increase in signal (increased NADH/NAD+ ratio) which is attributed to activation of the dehydrogenases of the TCA cycle by a high intramitochondrial [Ca2+]. The entire response was blocked by microinjection of the cell with Ruthenium Red (not shown). B, a rise in [Ca2+]c causes a transient mitochondrial depolarisation. Rat cortical astrocytes were loaded with tetramethyl-rhodamine ethyl ester (TMRE) and the [Ca2+]c indicator fluo-3, and imaged simultaneously on a confocal microscope (Zeiss 510CLSM). Application of ATP to a single cell raised [Ca2+]c (•) by IP3-dependent mobilisation from ER stores, and caused a small transient mitochondrial depolarisation (○), signalling mitochondrial Ca2+ uptake (an increase in TMRE fluorescence signals mitochondrial depolarisation – see Boitier et al. 1999). The same phenomenon is seen on a larger scale as [Ca2+]c waves are propagated through a network of interconnected astrocytes, as shown in C. Here, ATP application initiated a wave that propagated from cell to cell, imaged as described above. The steps from cell to cell are indicated by arrows. The simultaneous measurement of ΔΨm reveals a wave of mitochondrial depolarisation propagating through the network.
