Abstract
Protein extravasation and vasodilatation can be induced by neuropeptides released from nociceptive afferents (neurogenic inflammation). We measured electrically evoked neuropeptide release and concomitant protein extravasation in human and rat skin using intradermal microdialysis.
Plasmapheresis capillaries were inserted intradermally at a length of 1.5 cm in the volar forearm of human subjects or abdominal skin of rats. Capillaries were perfused with Ringer solution at a flow rate of 2.5 or 1.6 μl min−1. After a baseline period of 60 min capillaries were stimulated electrically (1 Hz, 80 mA, 0.5 ms or 4 Hz, 30 mA, 0.5 ms) for 30 min using a surface electrode directly above the capillaries and a stainless-steel wire inserted in the capillaries. Total protein concentration was assessed photometrically and calcitonin gene-related peptide (CGRP) and substance P (SP) concentrations were measured by enzyme-linked immunosorbent assay (ELISA).
In rat skin, electrical stimulation increased CGRP and total protein concentration in the dialysate. SP measurements showed a larger variance but only for the 1 Hz stimulation was the increased release significant.
In human skin, electrical stimulation provoked a large flare reaction and at a frequency of 4 Hz both CGRP and SP concentrations increased significantly. In spite of the large flare reactions no protein extravasation was induced, which suggests major species differences.
It will be of interest to investigate whether the lack of neurogenic protein extravasation is also valid under pathophysiological conditions.
Upon activation nociceptors release neuropeptides from their terminals in the central nervous system and in the periphery. In rodent skin neuropeptides induce a combination of vasodilatation and protein extravasation, which has been termed ‘neurogenic inflammation’ (Jancso et al. 1967). Neurogenic inflammation has been hypothesized to play an important pathophysiological role in diseases like migraine and asthma (Moskowitz, 1993). There is evidence from rat experiments that substance P (SP) is mainly active in inducing protein extravasation by activation of NK1 receptors, whereas calcitonin gene-related peptide (CGRP) is responsible for neurogenic vasodilatation (Holzer, 1998). This assumption has been partly confirmed in human skin where exogenous SP-induced protein extravasation (Hägermark et al. 1978; Jorizzo et al. 1983; Devillier et al. 1986) and CGRP provoked lasting and pronounced vasodilatation (Brain et al. 1985). However, release of endogenous SP by capsaicin does not induce protein extravasation (Schmelz et al. 1997a) or indirect release of histamine from mast cells (Tausk & Undem, 1995; Huttunen et al. 1996; Petersen et al. 1997b).
Neuropeptide concentrations in human skin are problematic to assess in humans in vivo (Hargreaves et al. 1994; Petersen et al. 1997b). In contrast, release models in rat skin have been successfully used to assess chemically or electrically induced neuropeptide release in vitro (Hua & Yaksh, 1992; Kilo et al. 1997; Kress et al. 1999).
The aim of this study was to enable direct comparison of the pattern of electrically evoked neurogenic inflammation by the use of experimental conditions as similar as possible to human and rat skin. For this purpose, we used identical dermal microdialysis systems for stimulation and measurement of protein and neuropeptides. Microdialysis is a minimally invasive technique that was originally developed for applications in the central nervous system (Ungerstedt & Hallstrom, 1987), but has also been adapted for dermal use (Anderson et al. 1991). For the purpose of this study a microdialysis membrane with a high cut-off of 3000 kDa was chosen to analyse protein extravasation (Schmelz et al. 1997a). Simultaneously, nociceptor activation can be assessed indirectly by conventional psychophysical methods and by analysis of the extent of the axon reflex erythema around the stimulated skin site.
METHODS
Subjects
Thirteen healthy volunteers aged 23-34 years (7 male; 6 female) participated in the experiments after having given written informed consent. All subjects were familiar with the principles of the method and the general intention of the study, but were unaware of the specific experimental goals. They could withdraw from the experiment at any time. The study was approved by the local ethics committee and was conducted according to the Declaration of Helsinki. The volunteers were comfortably seated on a reclining chair and their left arm was placed in a vacuum cushion with the volar side up. The arm was fixed to keep it in the same relaxed position during the whole experiment. Experimental sessions lasted about 3 h.
Animals
Experiments were performed under protocols approved by the local ethics committee (Ansbach, Germany). Thirty-four male Wistar rats (mean weight, 386 ± 11 g) were anaesthetized with an intraperitoneal injection of thiopental (120 mg kg−1). The abdomen was shaved with an electrical shaver. Skin temperature was held constant at 32°C by use of an infrared bulb that was feedback-controlled from a thermocouple attached to the abdomen (Physitemp, Clifton, NJ, USA). Adequate depth of anaesthesia was checked at regular intervals throughout the experimental procedure by firmly squeezing the tail. In case of the induction of nocifensive reflexes, an additional 10 mg thiopental was applied i.p. in a volume of 0.5 ml. Animals were killed by intracardial injection of 1 ml lidocaine (2 %) immediately after the end of the experimental procedure.
Microdialysis
The effects of electrical stimulation were analysed in humans and rats. Care was taken to use identical stimulation parameters and similar settings as far as possible. Four (1 Hz protocol) or six (4 Hz protocol) single plasmapheresis hollow fibres (Asashi, Japan; 0.4 mm diameter, cut-off 3000 kDa) were inserted intracutaneously for a length of 1.5 cm by a 25 gauge cannula in the abdominal skin of the rats or in the volunteers’ left volar forearm. The insertion of the thin needles was well tolerated by all volunteers without local anaesthesia. The insertion depth was measured by ultrasound (Dermascan C, Cortex Technologies, Denmark) to be 0.65 mm on average (range, 0.4-0.9 mm; n = 19). All hollow fibres were orientated transversally to the axis of the rat’s body or the human forearm. They were inserted in groups of two (1 Hz protocol) or three (4 Hz protocol) at a distance of 1-2 mm apart. The two or three groups of fibres were located 7 (human) or 4 cm (rat) apart to prevent mutual influence. The fibres were perfused with Ringer solution (Ringerlösung Fresenius, Germany) via Tygon tubing (Novodirect, Germany) and a microdialysis pump (Pump 22, Havard Apparatus, USA), on which four or six syringes (1 ml; Dispomed, Gelnhausen, Germany) were mounted and capped with 26 gauge hypodermic needles (Neoject, Hungary). For the 1 Hz protocol a flow rate of 2.5 μl min−1 was used. Because at this flow rate no CGRP increase was detected, the flow was reduced to 1.6 μl min−1 in the 4 Hz protocol to increase relative recovery. Increasing the number of capillaries per group to three instead of two ensured a sufficient amount of eluate. After passing through the skin the fibres were inserted into glass capillaries (150 μl; 1.0 mm inner diameter; Servoprax R, GLW, Germany) to collect the dialysate. Tilting the capillaries to an angle of 5 deg minimized outflow resistance. The air-exposed length of the microdialysis fibres was less than 3 mm on either the inflow or outflow side. The dialysate from each group of capillaries was collected in one common vial. Samples were taken every 30 min for a total period of 150 min and stored in polyethylene cups. They were immediately put on ice and separated for the different analyses.
Electrical stimulation
The plasmapheresis fibres, which were used for the electrical stimulation, were equipped with a stainless-steel wire (diameter 0.2 mm) in their lumen which acted as the cathode. They were electrically stimulated using a surface anode directly above the fibres (Fig. 1). To reduce the skin resistance electrode paste (electrode adhesive paste, Beckman, USA) was used. After 60 min of baseline measurement, constant current stimuli (0.5 ms, 80 mA, 1 Hz or 30 mA, 4 Hz) were applied for 30 min in rat or human skin from constant current stimulators (Digitimer Model DS7, UK). Each group of fibres was connected to a different stimulator. In each experiment one group of fibres was stimulated electrically. Control values were derived simultaneously from the non-stimulated group(s) of fibres. The dialysate from each group (stimulated and non-stimulated) was sampled separately. The stimulation was followed by a washout period of 60 min.
Figure 1. Schematic diagram of the experimental set-up.
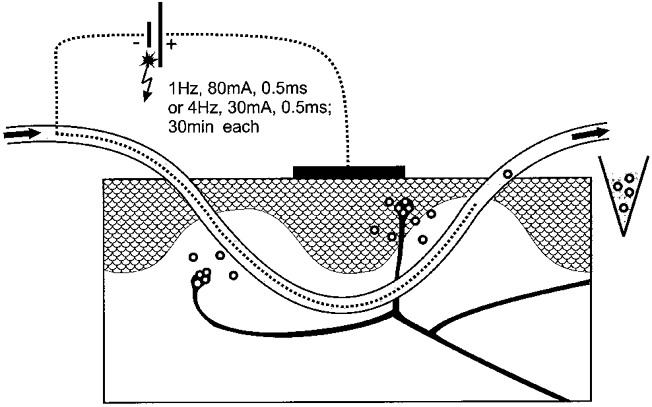
Note that two or three microdialysis fibres were inserted in parallel to increase relative recovery of the microdialysate.
Analysis
Each sample was analysed for CGRP, substance P and total protein. The neuropeptides were measured using the enzyme-linked immunosorbent assay (ELISA) technique (Cayman, USA; Spibio, France). Cross-reactivity of the CGRP ELISA to amylin was < 0.01 % and cross-reactivity of the SP ELISA to neurokinin A was 2.7 %. The protein extravasation (total protein content) was determined photometrically (MRX reader, Dynatech, Germany) using Coomassie Blue dye (Bradford, 1976) for the analysis and bovine serum albumin as a standard.
Psychophysics
In the human subjects, maximum pain sensations evoked by the electrical stimulation were assessed on a numeric scale from 0 to 10, in which the value of 0 indicated no sensation and 10 the maximum pain the subject could imagine.
Flare areas
The extent of the electrically evoked visible axon reflex erythema was marked on the forearm skin at its maximum size immediately after the stimulation period. At the end of the experiment the borders were traced on a transparent acetate sheet, digitized and the areas of the flare reaction were evaluated planimetrically using specialized software (Nischik & Forster, 1997).
Statistics
For statistical evaluation an ANOVA for repeated measures was calculated using stimulation frequency and treatment (stimulated/non-stimulated) as independent variables. Scheffé‘s post hoc test was used to identify significant differences. Pain ratings and flare sizes were compared using the non-parametric Mann-Whitney U test. P values less than 0.05 were considered significant. All values are given as means ± s.e.m. or median with quartiles given in parentheses as appropriate.
RESULTS
In humans, insertion of the fibres caused a small flare reaction (4.6 ± 1.1 cm2, n = 13) lasting ∼40 min. This was a slightly less intense reaction than that described previously by Anderson et al. (1994), which can be explained by the smaller diameter of the cannulae used in this study. At the end of the baseline period no visible erythema was left. Electrical stimulation induced a large visible axon reflex flare of 29.5 ± 3.1 cm2 at 1 Hz (n = 5) and 35.3 ± 2.1 cm2 at 4 Hz (n = 8). No significant difference could be determined between the flare sizes of the two frequencies.
The intensity of pain sensations did not differ significantly between the two stimulation frequencies. Maximum pain ratings were 7 (6, 7) for 1 Hz (n = 5) and 7 (6.5, 7) for 4 Hz (n = 8). Although being not far from the tolerance limit, all subjects completed the whole stimulation period of 30 min. The character of the sensation was described as purely painful by all subjects and had a sharp stinging and a burning component.
Neuropeptides
In the rat experiments the control values of CGRP remained at a constant baseline level of ∼21 pg ml−1 in the 1 Hz (n = 15) and 24 pg ml−1 in the 4 Hz protocol (n = 17) during the whole observation time. During the 1 Hz stimulation the CGRP release increased significantly by ∼50 % (n = 15; P < 0.01), whereas the corresponding control values remained constant. Electrical stimulation at 4 Hz increased the CGRP concentration in the dialysate by ∼250 % (n = 17; P < 0.001) compared with the controls in which the CGRP levels declined slightly. The CGRP increase provoked by the 4 Hz stimulation was significantly higher compared with the 1 Hz stimulation (P < 0.001). The CGRP concentration following the 4 Hz stimulation period remained at a significantly higher level (P < 0.05) 30 min after the end of stimulation (Fig. 2A).
Figure 2. Time courses of CGRP concentration in the dialysate of intradermal microdialysis fibres after stimulating (filled bar) at 4 Hz (n = 17 rats; n = 7 humans) and 1 Hz (n = 15 rats; n = 5 humans) in comparison to the control values in rat and human skin.
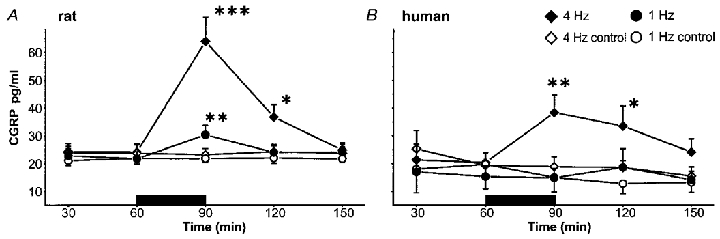
Because of the lower flow rate (1.6 and 2.5 μl min−1) CGRP levels at the 4 Hz stimulation are significantly higher for both species (P < 0.05). In rat (A), CGRP increase following the 4 Hz stimulation significantly exceeded that following the stimulation at 1 Hz and outlasted the stimulation period. In human skin (B), the stimulation at 1 Hz did not induce any increase in CGRP, whereas the stimulation at 4 Hz provoked a significant release of CGRP, which remained at a higher level during the whole observation time. Control values remained at a constant baseline level. At both frequencies CGRP levels in rat skin are significantly above those in human skin (P < 0.05). (***P < 0.001, **P < 0.01 and *P < 0.05; Scheffé‘s post hoc test.)
In human skin electrical stimulation at 1 Hz (n = 5) did not provoke a significant increase in CGRP release, whereas the 4 Hz stimulation (n = 7) increased the CGRP concentration by ∼100 % (P < 0.01). The corresponding control values decreased slightly. During the whole experiment non-stimulated values were measured at levels of ∼19 pg ml−1 (n = 7). Following the electrical stimulation at 4 Hz, the CGRP concentration remained elevated during the first washout interval (P < 0.05) compared with control (n = 7) (Fig. 2B).
CGRP levels in rat skin were significantly higher than in human skin following both stimulation frequencies (P < 0.05). According to the lower flow rate in the 4 Hz stimulation protocol (1.6 vs. 2.5 μl min−1) the CGRP concentrations were significantly higher compared with the 1 Hz protocol in rat and human (rat and human: P < 0.05).
In rat, electrical stimulation at 1 Hz evoked a slight, but significant release of substance P (n = 15; P < 0.01), whereas no increase was observed in the controls (n = 15). The non-stimulated substance P values remained stable at 16 pg ml−1 during the whole experimental time. The stimulation at 4 Hz evoked a similar increase in substance P (n = 13). However, because of higher variability this increase did not reach a significant level. In the controls substance P remained constant during the stimulation period (n = 13; Fig. 3A).
Figure 3. Time courses of substance P in rat (1 Hz, n = 15; 4 Hz, n = 13) and human (1 Hz, n = 5; 4 Hz, n = 7) skin.
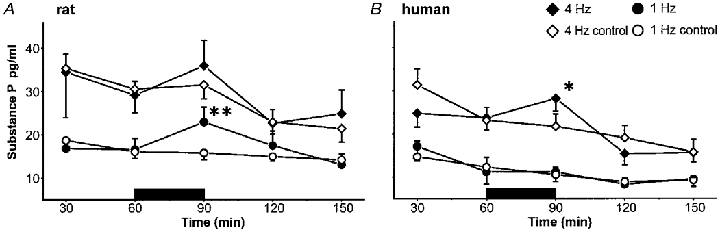
In rat skin significantly higher SP levels could be measured than in human skin (P < 0.05). According to the lower flow rate neuropeptide concentrations in the 4 Hz protocol are significantly higher compared with the 1 Hz protocol in both species (P < 0.001). In rat skin (A), a significant release of SP was observed during stimulation at 1 Hz, whereas the increase at the higher stimulation frequency did not reach a significant level. In contrast, in human skin (B) the stimulation at 1 Hz did not show any effects, whereas with the elevated stimulation frequency a significant SP release could be detected. Control values remained at an almost constant baseline level in both protocols. (**P < 0.01 and *P < 0.05; ANOVA, Scheffé‘s post hoc test.)
Similar to the CGRP results, no release of substance P was observed in human skin at a stimulation frequency of 1 Hz (n = 5). However, stimulation at the higher frequency (4 Hz; n = 7) significantly increased substance P levels in the dialysate (P < 0.05), whereas control levels declined slightly (Fig. 3B).
Substance P levels following electrical stimulation were found to be significantly higher in rat compared with human skin (P < 0.05). According to the lower flow rate in the 4 Hz stimulation protocol (1.6 vs. 2.5 μl min−1) the substance P concentrations were significantly higher compared with the 1 Hz protocol in rat and human (rat and human: P < 0.001).
Protein extravasation
Total protein content in rat and human skin increased with reduced flow as expected. Significantly higher levels were measured in the 4 Hz protocols (1.6 μl min−1) compared with the 1 Hz protocols (2.5 μl min−1) (rat, P < 0.001; human, P < 0.05).
In rat, the total protein concentration in the dialysate in the stimulated and the non-stimulated control capillaries decreased exponentially during the first hour. In the 1 Hz protocol it declined from ∼0.75 to 0.45 mg ml−1 60 min later (n = 15). In the 4 Hz protocol the protein content dropped from ∼1.0 to 0.6 mg ml−1 (n = 17). While the total protein concentration in the control area steadily declined, electrical stimulation evoked a significant increase in total protein at the stimulation site. Total protein significantly increased during stimulation at 1 Hz (n = 15; P < 0.001) and 4 Hz (n = 17; P < 0.001). The total protein levels remained significantly elevated at 30 min (P < 0.01) and 60 min (P < 0.05) after the end of the 4 Hz stimulation. In the 1 Hz experiments only the first washout value was significantly elevated compared with the control value (P < 0.01) (Fig. 4A).
Figure 4. Protein extravasation in electrically stimulated and non-stimulated capillaries in rats (1 Hz, n = 15; 4 Hz, n = 17) and humans (1 Hz, n = 5; 4 Hz, n = 8).
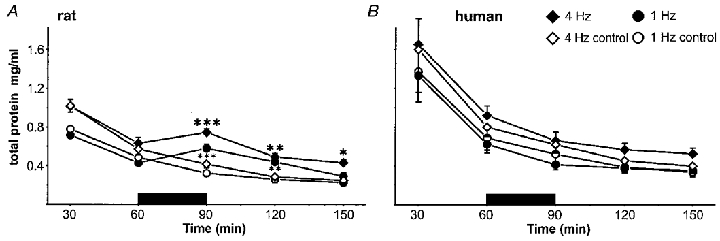
In rat skin (A), total protein content exponentially declined after insertion of the microdialysis fibres to a baseline level, from which electrical stimulation evoked a significant increase of protein at both 1 and 4 Hz. In contrast, protein content in the dialysate derived from the non-stimulated capillaries continued to decrease gradually. Following 4 Hz stimulation protein extravasation was elevated during the complete sampling period, whereas at the 1 Hz stimulation only the first washout value was significantly increased. In human skin (B), plasma extravasation exponentially decreased in the stimulated and non-stimulated capillaries at both frequencies. In both species the 4 Hz protein values were significantly elevated compared with the 1 Hz values due to the lower perfusion rate (rat skin, P < 0.001; human skin, P < 0.05). (***P < 0.001, **P < 0.01 and *P < 0.05, ANOVA, Scheffé‘s post hoc test.)
In human skin the protein extravasation exponentially decreased in the dialysate collected from the stimulated and non-stimulated capillaries at both frequencies. Following electrical stimulation at 1 Hz total protein declined from ∼0.65 to 0.45 mg ml−1 (n = 5), and no significant differences were observed between stimulated and non-stimulated sites. A similar pattern was observed at both 4 Hz stimulation (n = 8) and the subsequent washout periods (Fig. 4B).
DISCUSSION
Electrically evoked neurogenic inflammation in rat skin
Antidromic stimulation of nociceptive afferent fibres classically provokes a combination of vasodilatation and protein extravasation (Jancso et al. 1967). For the assessment of protein extravasation following electrical stimulation of peripheral nerves or dorsal roots (Pinter & Szolcsanyi, 1995) the Evans Blue technique has traditionally been used. Our results on electrically induced protein extravasation confirm earlier studies showing effects at low stimulation frequencies using antidromic nerve stimulation (Szolcsanyi, 1996). Similarly, dose-dependent CGRP release upon chemical (Kilo et al. 1997) or electrical stimulation (Hua & Yaksh, 1992; Kress et al. 1999) has been reported before in in vitro systems.
Electrically evoked neurogenic inflammation in human skin
In pig skin mechano-insensitive nociceptors have been found to be responsible for the axon reflex erythema (Lynn et al. 1996). In human skin these mechano-insensitive, but chemosensitive, nociceptors have been implicated in the axon reflex flare (Schmelz et al. 2000a). Interestingly, thresholds for transcutaneous stimulation of these fibres have been found to be unexpectedly high (30-60 mA) when compared with conventional mechanoresponsive ‘polymodal’ nociceptors (< 10 mA) (Weidner et al. 1999). This fact might explain why in human subjects even painful electrical stimulation failed to elicit a visible flare reaction comparable to the large axon reflex erythema flare provoked by chemical stimulation, but the flare could be detected by laser Doppler techniques (Magerl et al. 1987; Westerman et al. 1987). In our study electrical stimulation at a very high intensity was applied. The resulting flare reaction was intense and of similar size to the axon reflex erythema provoked by intense chemical stimulation such as intradermal injection of 100 μg capsaicin (Simone et al. 1989) (32 cm2) or 100 μg histamine (21 cm2) (Simone et al. 1987). This result suggests that widely branched chemonociceptors were excited by the intense electrical stimulation. Thus, our results are consistent with the high electrical threshold, large innervation territories and sustained responses upon stimulation with capsaicin or histamine (Schmelz et al. 1997b; Schmelz et al. 2000b) of mechano-insensitive nociceptors.
Electrical stimulation at both frequencies provoked a large axon reflex erythema but only at 4 Hz could a significant increase in CGRP be measured. This discrepancy might be explained best by the lower flow rate and higher number of microdialysis capillaries (3 instead of 2) used in the 4 Hz protocol. Lower flow rate and larger surface area will increase recovery and therefore the sensitivity of the method increases. The time course of CGRP increase reflects the longer half-life of this neuropeptide in the skin (Brain et al. 1985; McEwan et al. 1988).
Stimulated SP increases have been problematic to assess in non-neuronal tissues of humans (Schmelz et al. 1997a; Petersen et al. 1997a) and animals (Hua & Yaksh, 1992; Kress et al. 1999; Ebersberger et al. 1999). Using low flow rates and three parallel microdialysis capillaries we succeeded in measuring SP increases provoked by electrical stimulation. However, the increases were only moderate and showed greater variance than the CGRP data. These results might reflect the high prevalence of CGRP-positive dermal nerve fibres in human skin compared with SP-positive fibres (Wallengren et al. 1987; Chan et al. 1997; Schulze et al. 1997).
Although electrical stimulation provoked massive axon reflex erythema and clear neuropeptide release, no neurogenic protein extravasation was detected. Dermal microdialysis has been shown to be a very sensitive method of detecting protein extravasation and by far exceeds the sensitivity of visual wheal formation (Weidner et al. 2000). Moreover, in rat skin there was a pronounced protein extravasation that we assessed using the same technique. Therefore, the lack of neurogenic protein extravasation in human skin cannot be attributed to limitations of the microdialysis technique. Our findings corroborate studies showing unchanged protein extravasation following capsaicin stimulation (Schmelz et al. 1997a) in human and also pig skin (Pierau et al. 1994). There is also no evidence for an indirect mechanism of neurogenic protein extravasation in human skin via histamine release from SP-activated mast cells (Tausk & Undem, 1995; Huttunen et al. 1996; Petersen et al. 1997a; Schmelz et al. 1999).
Implication for species differences
The basic mechanism underlying the differences between neurogenic protein extravasation in rodents and in humans is, as yet, unclear. Theoretically, lower SP concentrations, faster SP breakdown, fewer NK1 receptors and/or longer distance from the nociceptive nerve terminals to the vessels could be of importance. Exogenously applied SP is also capable of inducing protein extravasation in human skin. When applied via microdialysis capillaries vasodilatation and protein extravasation are elicited at concentrations of 10−8 M without concomitant release of histamine (Weidner et al. 2000) suggesting there is no lack of functional NK1 receptors in human skin. There is also evidence that rat organs with lower SP content, such as muscle, do not exhibit neurogenic protein extravasation (McMahon et al. 1984). Significantly lower SP concentrations in human skin as found in our study could thus contribute to the lack of protein extravasation. In accordance with our data, the total SP concentration in human forearm skin was lower than in abdominal skin of adult rat (Eedy et al. 1991, 1994). In addition, CGRP-positive fibres around vessels in human skin were found to co-localize especially somatostatin, but not SP (Gibbins et al. 1987). Thus, for SP the diffusion distance to the vessels might be larger.
In summary, transcutaneous electrical stimulation provoked neuropeptide release and vasodilatation in rat and human skin, whereas neurogenic protein extravasation was only observed in rat skin. It can be speculated whether this difference may offer a reason for the failure of NK1 antagonists in the treatment of migraine (Roon et al. 2000), although they potently inhibit electrically evoked protein extravasation in rat dura mater (Polley et al. 1997). In accordance with our results, no increase in fluorescein-marked retinal protein extravasation could be detected in acute migraine, whereas electrical stimulation of the trigeminal ganglion evoked massive retinal protein extravasation in rat (May et al. 1998).
However, the lack of neurogenic protein extravasation in healthy human skin or in the retina during a migraine attack does not exclude a role for neuropeptides under pathological conditions. Higher SP concentrations have been suggested to be a relevant pathophysiological factor for the vasodilatation, oedema and trophic disturbances seen in patients suffering from complex regional pain syndrome (M. Sudeck, reflex sympathetic dystrophy) (Blair et al. 1998). Indeed, transcutaneous electrical stimulation with identical parameters to those in the present study provoked enhanced protein extravasation in this patient group, which was also accompanied by an enhanced flare reaction (Weber et al. 2000). Thus, although neurogenic protein extravasation is absent in healthy human skin, it may well be an important factor under pathophysiological conditions.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 353).
References
- Anderson C, Andersson T, Molander M. Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Dermato-Venereologica. 1991;71:389–393. [PubMed] [Google Scholar]
- Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. Journal of Investigative Dermatology. 1994;102:807–811. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta Orthopaedica Belgica. 1998;64:448–451. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Archives of Dermatological Research. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- Devillier P, Regoli D, Asseraf A, Descours B, Marsac J, Renoux M. Histamine release and local responses of rat and human skin to substance P and other mammalian tachykinins. Pharmacology. 1986;32:340–347. doi: 10.1159/000138190. [DOI] [PubMed] [Google Scholar]
- Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- Eedy DJ, Shaw C, Johnston CF, Armstrong EP, Buchanan KD. Neuropeptides of the primary sensory neurones in rat skin: an ontogenic study. Regulatory Peptides. 1991;33:175–182. doi: 10.1016/0167-0115(91)90211-x. [DOI] [PubMed] [Google Scholar]
- Eedy DJ, Shaw C, Johnston CF, Buchanan KD. The regional distribution of neuropeptides in human skin as assessed by radioimmunoassay and high-performance liquid chromatography. Clinical and Experimental Dermatology. 1994;19:463–472. doi: 10.1111/j.1365-2230.1994.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Wattchow D, Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Research. 1987;414:143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- Hägermark O, Hokfelt T, Pernow B. Flare and itch induced by substance P in human skin. Journal of Investigative Dermatology. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Swift JQ, Roszkowski MT, Bowles W, Garry MG, Jackson DL. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surgery, Oral Medicine and Oral Pathology. 1994;78:503–510. doi: 10.1016/0030-4220(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. General Pharmacology. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Hua XY, Yaksh TL. Release of calcitonin gene-related peptide and tachykinins from the rat trachea. Peptides. 1992;13:113–120. doi: 10.1016/0196-9781(92)90148-v. [DOI] [PubMed] [Google Scholar]
- Huttunen M, Harvima IT, Ackermann L, Harvima RJ, Naukkarinen A, Horsmanheimo M. Neuropeptide- and capsaicin-induced histamine release in skin monitored with the microdialysis technique. Acta Dermato-Venereologica. 1996;76:205–209. doi: 10.2340/0001555576205209. [DOI] [PubMed] [Google Scholar]
- Jancso N, Jancso GA, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. British Journal of Pharmacology. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorizzo JL, Coutts AA, Eady RA, Greaves MW. Vascular responses of human skin to injection of substance P and mechanism of action. European Journal of Pharmacology. 1983;87:67–76. doi: 10.1016/0014-2999(83)90051-1. [DOI] [PubMed] [Google Scholar]
- Kilo S, HardingRose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kress M, Guthmann C, Averbeck B, Reeh PW. Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin, in vitro. Neuroscience. 1999;89:303–310. doi: 10.1016/s0306-4522(98)00280-2. [DOI] [PubMed] [Google Scholar]
- Lynn B, Schutterle S, Pierau FK. The vasodilator component of neurogenic inflammation is caused by a special subclass of heat-sensitive nociceptors in the skin of the pig. The Journal of Physiology. 1996;494:587–593. doi: 10.1113/jphysiol.1996.sp021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan JR, Benjamin N, Larkin S, Fuller RW, Dollery CT, MacIntyre I. Vasodilatation by calcitonin gene-related peptide and by substance P: a comparison of their effects on resistance and capacitance vessels of human forearms. Circulation. 1988;77:1072–1080. doi: 10.1161/01.cir.77.5.1072. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Sykova E, Wall PD, Woolf CJ, Gibson SJ. Neurogenic extravasation and substance P levels are low in muscle as compared to skin the rat hindlimb. Neuroscience Letters. 1984;52:235–240. doi: 10.1016/0304-3940(84)90167-8. [DOI] [PubMed] [Google Scholar]
- Magerl W, Szolcsanyi J, Westerman RA, Handwerker HO. Laser Doppler measurements of skin vasodilation elicited by percutaneous electrical stimulation of nociceptors in humans. Neuroscience Letters. 1987;82:349–354. doi: 10.1016/0304-3940(87)90281-3. [DOI] [PubMed] [Google Scholar]
- May A, Shepheard SL, Knorr M, Effert R, Wessing A, Hargreaves RJ, Goadsby PJ, Diener HC. Retinal plasma extravasation in animals but not in humans: implications for the pathophysiology of migraine. Brain. 1998;121:1231–1237. doi: 10.1093/brain/121.7.1231. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43:S16–20. [PubMed] [Google Scholar]
- Nischik M, Forster C. Analysis of skin erythema using true color images. IEEE Transactions on Medical Imaging. 1997;16:711–716. doi: 10.1109/42.650868. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Church MK, Skov PS. Histamine is released in the wheal but not the flare following challenge of human skin in vivo: a microdialysis study. Clinical and Experimental Allergy. 1997a;27:284–295. doi: 10.1046/j.1365-2222.1997.d01-502.x. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Winge K, Brodin E, Skov PS. No release of histamine and substance P in capsaicin-induced neurogenic inflammation in intact human skin in vivo: a microdialysis study. Clinical and Experimental Allergy. 1997b;27:957–965. [PubMed] [Google Scholar]
- Pierau FK, Ernst R, Faulstroh K, Sann H, Donnerer J. Neurogenic inflammation in the pig’s skin: flare reaction and plasma extravasation. In: Hökfelt T, Schaible HG, Schmidt RF, editors. Neuropeptides, Nociception, and Pain. Weinheim: Chapman & Hall; 1994. pp. 85–102. [Google Scholar]
- Pinter E, Szolcsanyi J. Plasma extravasation in the skin and pelvic organs evoked by antidromic stimulation of the lumbosacral dorsal roots of the rat. Neuroscience. 1995;68:603–614. doi: 10.1016/0306-4522(95)00104-q. [DOI] [PubMed] [Google Scholar]
- Polley JS, Gaskin PJ, Perren MJ, Connor HE, Ward P, Beattie DT. The activity of GR205171, a potent non-peptide tachykinin NK1 receptor antagonist, in the trigeminovascular system. Regulatory Peptides. 1997;68:23–29. doi: 10.1016/s0167-0115(96)00137-1. [DOI] [PubMed] [Google Scholar]
- Roon KI, Olesen J, Diener HC, Ellis P, Hettiarachchi J, Poole PH, Christianssen I, Kleinermans D, Kok JG, Ferrari MD. No acute antimigraine efficacy of CP-122,288, a highly potent inhibitor of neurogenic inflammation: results of two randomized, double-blind, placebo-controlled clinical trials. Annals of Neurology. 2000;47:238–241. [PubMed] [Google Scholar]
- Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neuroscience Letters. 1997a;230:1–4. doi: 10.1016/s0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schmidt R, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? NeuroReport. 2000a;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. Journal of Neuroscience. 1997b;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000b;123:560–571. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Zeck S, Raithel M, Rukwied R. Mast cell tryptase in dermal neurogenic inflammation. Clinical and Experimental Allergy. 1999;29:652–659. doi: 10.1046/j.1365-2222.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- Schulze E, Witt M, Fink T, Hofer A, Funk RH. Immunohistochemical detection of human skin nerve fibers. Acta Histochemica. 1997;99:301–309. doi: 10.1016/S0065-1281(97)80024-4. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Ngeow JY, Whitehouse J, Becerra Cabal L, Putterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosensensory Research. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Neurogenic inflammation: reevaluation of the axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. Boca Raton: CRC Press; 1996. pp. 33–40. [Google Scholar]
- Tausk F, Undem B. Exogenous but not endogenous substance P releases histamine from isolated human skin fragments. Neuropeptides. 1995;29:351–355. doi: 10.1016/0143-4179(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Hallstrom A. In vivo microdialysis – a new approach to the analysis of neurotransmitters in the brain. Life Sciences. 1987;41:861–864. doi: 10.1016/0024-3205(87)90181-0. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Ekman R, Sundler F. Occurrence and distribution of neuropeptides in the human skin. An immunocytochemical and immunochemical study on normal skin and blister fluid from inflamed skin. Acta Dermato-Venereologica. 1987;67:185–192. [PubMed] [Google Scholar]
- Weber M, Birklein F, Neundørfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2000. in the Press. [DOI] [PubMed]
- Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of SP and CGRP in human skin – a microdialysis study. Journal of Investigative Dermatology. 2000. in the Press. [DOI] [PubMed]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. Journal of Neuroscience. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman RA, Low A, Pratt A, Hutchinson JS, Szolcsanyi J, Magerl W, Handwerker HO, Kozak WM. Electrically evoked skin vasodilatation: a quantitative test of nociceptor function in man. Clinical and Experimental Neurology. 1987;23:81–89. [PubMed] [Google Scholar]


