Figure 2.
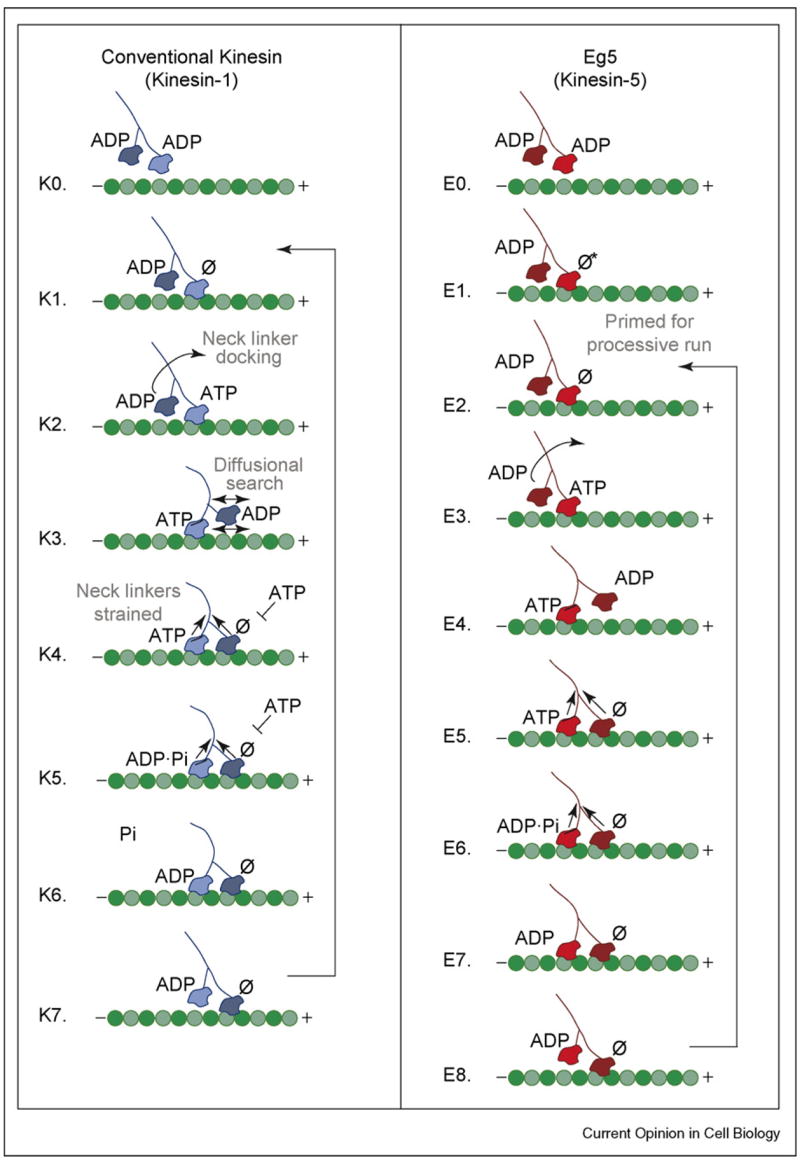
Stepping models for Kinesin and Eg5. For kinesin, a processive run begins when a motor head collides with the microtubule and releases its ADP. ATP binding at the forward head leads to neck linker docking and plus-end directed movement of the rearward head that promotes binding at the next site on the microtubule. Release of ADP at step K4 results in a state with both heads bound tightly to the microtubule and the development of intermolecular strain between the two motor domains. ATP hydrolysis occurs on the rearward head followed by phosphate release and rearward head detachment, which returns the motor to K1, having taken a single step. For kinesin, phosphate release is rate-limiting. In contrast to kinesin, Eg5 collides with the microtubule but cannot bind ATP until a conformational change occurs, which is believed to involve movement of the neck linker. This isomerization establishes a processive run (step E2), allowing ATP binding on the forward head. Successive steps in the cycle are similar to those for kinesin but with rate-limiting ATP hydrolysis.
