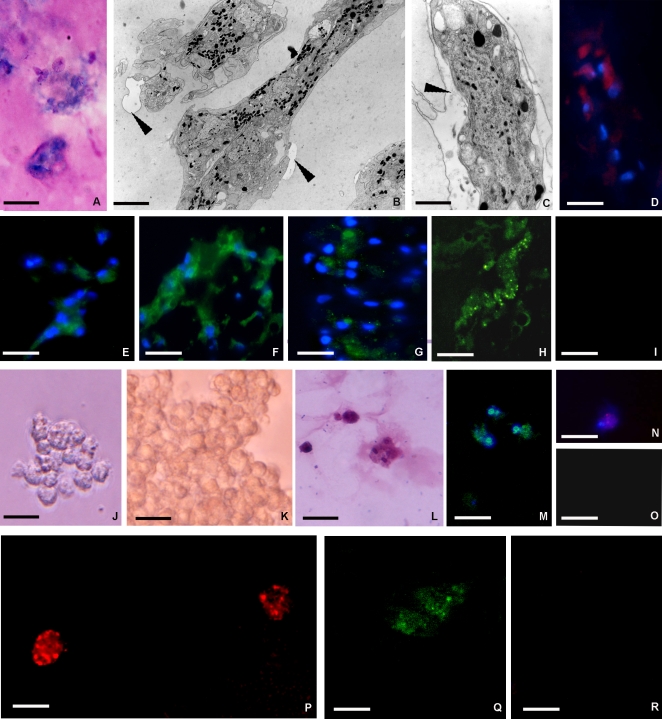
Figure 3. Analysis of the phenotype of cells recruited into the matrigel sponges by MCP-1/CCL2.
MCP-1/CCL2 induces the mobilization of cells from tissues surrounding the MG implant into the biopolymer that was recovered and processed for structural, ultrastructural immunocytochemical analysis or seeded for cell culture (A–O). After 1 week in vivo the matrigel implant contained granular cells that stained with Giemsa solution (A) that were ultrastructurally similar (B) to those localized in the extracellular matrix (ECM) in stimulated leeches (Fig. 2C, arrowhead). These cells showed a cytoplasm filled with black granules (B, C). Again they were of a migratory phenotype with localized degradation of ECM (B, C arrowheads), and cathepsin B staining (D). Immunocytochemical characterization of cells infiltrating the MG supplemented with MCP-1/CCL2 (E–I). The cells expressed several markers typical of granulocytes: CD11c (E), CD14 (F), CD61 (G), CD68 (H); (I: negative control). After 1 week in vivo the MG was used to seed cell cultures (J–O). Phase-contrast images of granular cells obtained 3 days (J) and 1 week (K) post-seeding indicate increasing cell numbers (K). These Giemsa positive cells (L) also immuno-stained with a CD11c antibody (M), a marker of granulocytes, and with anti-BrdU (N) demonstrating that these cells attraversed S-phase in culture; O: negative control. After incubation with Dil-Ac-LDL, these cells also took up the fluorochrome-conjugated Ac-LDL (P). These results strongly indicate that the cells that migrated out of the matrigel in vitro consisted largely of cells maintaining a monocyte/macrophage function throughout in vivo and in vitro culture as indicated by expression of CD68 (Q, R). Bars: A, D, P–R 10 µm; B, C 1 µm; E–I 15 µm; J–O 20 µm