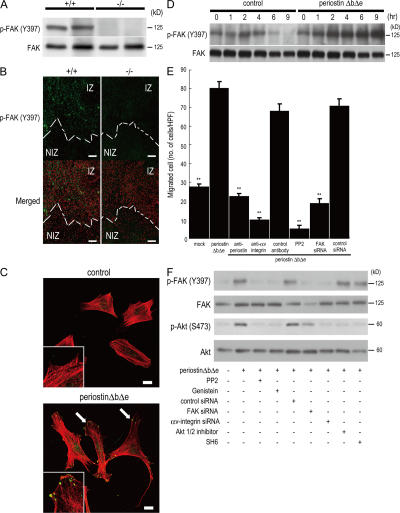
Figure 4.
Periostin promotes cell migration through integrin-mediated FAK signaling. (A) Phosphorylation of FAK in infarct LV from periostin +/+ mice and −/− mice 5 d after AMI. (B) Immunofluorescence for phosphorylated FAK (p-FAKY397) in the border of infarct LV from periostin +/+ mice and −/− mice 5 d after AMI. Merged images show an overlay of p-FAKY397 (green) and propidium iodide–stained nuclei (red). The dotted line shows the infarct border. NIZ, noninfarct zone; IZ, infarct zone. (C and D) Promotion of cell spreading and activation of FAK phosphorylation in vitro. The morphology of starved C3H10T1/2 cells was analyzed by immunofluorescence 12 h after adding periostin ΔbΔe (C), and the p-FAKY397 was examined by Western blot analysis at various times after adding periostin ΔbΔe (D). In C, the merged images show an overlay of p-FAKY397 (green) and rhodamine-phalloidin (red), and the arrows point to FAK phosphorylation sites. The insets show higher magnification of the cell processes. (E) Chemotaxis of primary cardiac fibroblasts from periostin −/− mice in the absence (mock) or presence of periostin ΔbΔe, detected by an in vitro cell migration assay. Cardiac fibroblasts were significantly activated by periostin ΔbΔe, and treatment with neutralizing antibodies against periostin and αv-integrin, PP2, or FAK siRNAs reduced the cell migration. **, P < 0.001 vs. periostin ΔbΔe. Error bars represent the mean ± the SEM. (F) Periostin can stimulate FAK and Akt phosphorylation through integrin signaling. Starved C3H10T1/2 cells were incubated for 1 h with periostin ΔbΔe with or without each siRNA or the FAK and Akt inhibitors. Bars: (B) 100 μm; (C) 20 μm.