Abstract
To understand lens fiber cell elongation- and differentiation-associated cytoskekeletal remodeling, here we identified and characterized the major protein components of lens fiber cell triton X-100 insoluble fraction by mass spectrometry and immunoblot analysis. This analysis identified spectrin, filensin, vimentin, tubulin, phakinin and β-actin as major cytoskeletal proteins in the lens fibers. Importantly, ezrin, radixin and moesin (ERM), heat-shock cognate protein 70, and β/γ-crystallins were identified as major cytoskeletal-associated proteins. ERM proteins were confirmed to exist as active phosphorylated forms that exhibited intense distribution in the organelle free-zone fibers. Furthermore, ERM protein phosphorylation was found to be dramatically reduced in Rho GTPase-targeted transgenic mouse lenses. These data identify the ERM proteins, which crosslink the plasma membrane and actin, as major and stable cytoskeletal-associated proteins in lens fibers, and indicate a potential role(s) for the ERMs in fiber cell actin cytoskeletal and membrane organization.
Keywords: Lens fibers, Triton Cytoskeleton, ERM proteins, Differentiation and Rho GTPases
The transparent vertebrate lens which plays a critical role in vision by focusing the incident light onto retina, is a unique avascular tissue composed of a single cell type of epithelial cells with different stages of differentiation. The cuboidal cells at the equatorial region of the lens epithelium divide and differentiate into elongated ribbon-like fibers on a continuous basis throughout the life. As these epithelial cells start differentiating into fiber cells, they exit from cell cycle, elongate progressively and migrate inward by making contacts posteriorly with the lens capsule and anteriorly with the epithelium through their anterior and posterior terminals, respectively [1, 2]. During differentiation, these fiber cells express and accumulate several lens abundant proteins called crystallins, lens specific cytoskeletal beaded filament proteins (filensin and phakinin), and transmembrane proteins including water channel (aquaporins) and gap junction (connexins) proteins [2]. Finally, during terminal differentiation, the fiber cells lose most cellular organelles including the nuclei, in a programmed manner evolving into prismoid fiber cells that organize with perfect symmetry [2, 3]. Lens fiber cell morphology, migration, membrane remodeling and intercellular adhesions are considered to be some of the key determinants of lens shape, polarity and ultimately, optical properties [2]. During differentiation, lens fiber cells undergo extensive membrane remodeling, including development of unique ball and socket interlocking digitations and fusion of lateral fiber cell membranes [4]. Concomitantly, the lens fiber cell actin cytoskeleton, membrane skeleton and cell-cell adhesion complexes reorganize in a distinct manner during elongation and differentiation[5-8]. Lens fiber cell membrane transport activity, including movement of water, ions and small molecules, is critical for lens transparency, and mutations in crystallins, aquaporins, connexins and cytoskeletal proteins are associated with cataractogenesis, indicating the importance of membrane architecture and transport activity for lens function [2, 9]. Although it is well recognized that actin cytoskeletal reorganization, mechanical properties, cell-cell adhesions and membrane remodeling play crucial roles in lens fiber cell elongation and differentiation [2], the cellular pathways regulating these events are poorly understood.
Recent studies involving functional disruption of cytoskeletal regulatory signaling molecules using gene targeting approaches, have revealed that the Rho family of small GTPases [10, 11], and the c-abl kinase-interacting proteins[12] play a pivotal role in lens fiber cell migration, elongation and transparency. To obtain further insight into the pathways/mechanisms regulating lens cytoskeletal reorganization during lens development and growth, we have isolated and characterized the protein profile of the lens fiber cell triton cytoskeleton (Triton X-100 insoluble fraction), with an emphasis on identifying the predominant cytoskeletal and cytoskeletal-associated proteins.
Materials and Methods
Lens dissection
Freshly extracted mouse lenses from one month-old BL6 mice were microdissected to separate the epithelium and fiber cell mass using a dissecting microscope (Zeiss Stemi 2000, Germany). The dissected tissue fractions were immediately frozen on dry ice. Similarly, freshly obtained porcine eyes (from a local slaughter house) were dissected to extract the lens, and after removing the capsule/epithelium, the lens outer cortical fiber mass was microdissected. Transgenic mice expressing C3 exoenzyme, an ADP-ribosylating RhoGTPase inactivating enzyme, or Rho GDP dissociation inhibitor (RhoGDIα) in a lens-specific manner, which were developed and maintained as described earlier by us [10, 11], were euthanized and dissected in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research, under an approved Duke University institutional animal protocol.
Extraction of Triton insoluble fraction
The lens fiber tissue (mouse and porcine) was homogenized in solubilization buffer (SB) containing 10 mM Piperazine-N,N-bis[2-ethanesulfonic acid] dipotassium salt, 50 mM KCl, 10 mM Ethylene glycol-bis (β-aminoethyl ether) N,N,N,N-tetracetic acid (EGTA), 3 mM MgCl2, 2 M glycerol , 2 mM NaF, 1 mM Na3VO4 and protease inhibitors (25μg/ml each of aprotinin, leupeptin and pepstatin), using a Dounce glass homogenizer with glass pestle. Tissue homogenates were centrifuged for 15 minutes at 800×g. The resulting supernatants were then centrifuged at 35,000×g for 30 minutes, after which, the pellets obtained were washed in SB and re-homogenized in SB containing 1% Triton X-100. The detergent treated samples were then centrifuged at 35,000×g for 30 minutes and the insoluble cytoskeletal fraction (pellet) was washed once again and suspended in extraction buffer containing 20 mM Tris–HCl, 300 mM NaCl, 30 mM MgCl2, 1 mM EGTA, 1 mM 1,4 Dithiothreitol (DTT) and protease inhibitors. All procedures were performed at 4 °C. Protein concentration was determined by Bio-Rad Dye Reagent procedure.
Electrophoresis and In-gel trypsin digestion
The triton-insoluble lens protein fractions were separated on sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) containing 5.5, 8, 10, or 12 % acrylamide. Protein samples were dissolved in 2x Laemmli loading solution and boiled for two minutes before analysis by SDS-PAGE. After electrophoresis, SDS-PAGE gels were rinsed with pure water and stained overnight at room temperature, using the GelCode Blue stain reagent (Pierce, Rockford, IL). Following this, the gels were destained and distinctly stained protein bands were excised from the gels using carbon steel surgical blades and a light box. Gel slices containing protein bands were subjected to in-gel tryptic digestion using the In-Gel Tryptic digestion kit (Pierce), per the manufacturer’s instructions. This digestion process included reduction and alkylation of protein samples.
Mass spectrometry
Trypsin digested peptide-matrix mixes were subjected to a combination of peptide mass finger printing analyses (Matrix-assisted laser desorption/ionisation-time of flight-time of flight mass spectrometry-MALDI-TOF MS) and MS/MS (Tandem mass spectrometry), using the 4700 Proteomics analyzer and GPS software package (Applied Biosystems, Foster City, CA). Proteins were identified based on MS and MS/MS spectra using the Mascot search engine. Collision-induced dissociation spectra were submitted for protein identification with a precursor precision tolerance of 0.1 Da and MS/MS fragment tolerance of 0.5 Da. Where indicated, the proteins from porcine species were identified using a mammalian database (The National Center for Biotechnology Information, NCBI).
Immunoblotting
To confirm identity of lens fiber cell triton cytoskeletal proteins initially identified by mass spectrometry analysis, samples of lens triton cytoskeletal fractions were separated by SDS-PAGE (8% and 12% acrylamide), transferred to nitrocellulose, blocked with 3% non-fat milk protein, and immunoblotted using appropriate primary and secondary antibodies following standard procedures. Similarly, whole tissue extracts were prepared from lenses of neonatal transgenic mice expressing C3 exoenzyme and Rho GDIα, using hypotonic buffer containing 10 mM Tris buffer pH 7.4, 0.2 mM MgCl2, 5 mM N-ethylmaleimide, 2 mM Na3VO4, 10 mM NaF, 60 μM phenyl methyl sulfonyl fluoride (PMSF), 0.4 mM iodoacetamide, 0.3μM aprotinin, 5.0 μM pepstatin and 4.0μM leupeptin. Lens insoluble extracts (100,000×g pellets) were then assayed for changes in ERM protein phosphorylation by immunoblot analysis. Polyclonal Phospho-Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558) antibody and Hsc-70 antibodies were obtained from Cell Signaling (Danvers, MA) and Stressgene (Ann Arbor, MI), respectively. β and γ-crystallin polyclonal antibodies were kindly provided by Samuel Zigler (Johns Hopkins School of Medicine). Immunoblots were developed with either enhanced chemiluminescence (ECL) reagent (Amersham, Piscataway, NJ) or with a horseradish peroxidase colorimetric reagent (KPL, Gaithersburg, MD).
Immunofluorescence
Cryostat sections were derived from neonatal mouse lenses (7 μm, sagittal and equatorial planes) as described earlier [11]. Air-dried sections were immunostained with polyclonal antibody raised against phospho-ERMs in conjunction with Alexa Fluor® 488 goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) as described earlier [11], and viewed under a Zeiss LSM 410 confocal microscope. For actin staining, the pre-blocked sections were labeled with phalloidin conjugated with tetra rhodamine isothiocyanate (Sigma-Aldrich, St. Louis, MO), as described above. Mouse lens primary epithelial cells cultured on glass cover slips were immunostained for phospho-ERM and photographed as described earlier [13].
Results
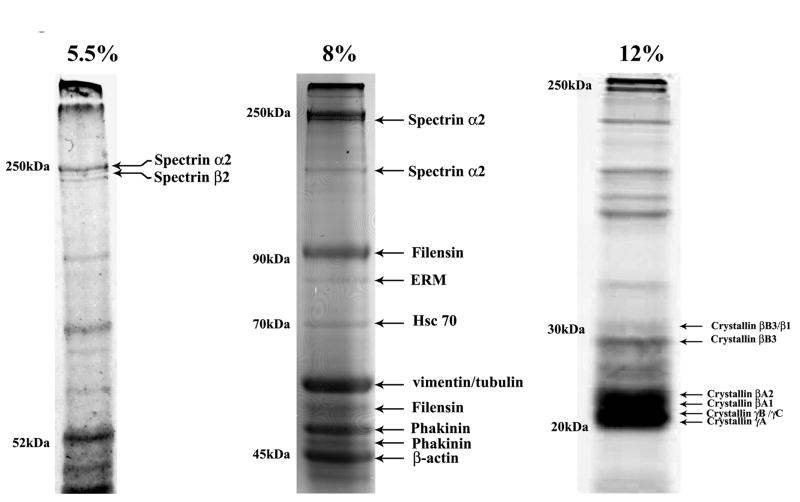
To obtain broader understanding of the interrelationship between lens fiber cell elongation/differentiation and cytoskeletal remodeling, this study was focused on identifying and characterizing the major protein components of the triton insoluble fraction of differentiating lens fiber cells. SDS-PAGE analysis of Triton X-100 insoluble protein fractions derived from mouse and porcine lens fiber cells revealed similar profiles, comprised of several distinctly resolved proteins with apparent molecular weights ranging between 20 kDa and > 250 kDa (Fig. 1). Polypeptides derived from in-gel trypsin digests of the various protein bands were identified by MALDI-TOF-TOF Mass spectrometry. This analysis identified α and β-spectrin, filensin (also identified as CP-94), vimentin/tubulin, phakinin (CP-49), β-actin, β-crystallin (βA2, βA1, βB3, and βB1), γ-crystallin (γB, γA and γC), proteolytically cleaved spectrin, CP-94 and CP-49, as some of the major proteins in the triton insoluble fraction (Fig. 1). In addition to these major components, two prominent protein bands with molecular mass of ≈ 80 and 70 kDa were also detected. The 80 kDa protein band was identified as a mixture of ezrin, radixin and moesin, while the 70 kDa protein was confirmed to be the heat-shock cognate protein 70 (Hsc70) by MALDI-TOF-TOF Mass spectrometry analysis (Fig. 1). To identify proteins with apparent molecular mass greater than 200 kDa, the triton insoluble protein samples were separated on SDS-polyacrylamide gels containing 5.5% acrylamide. These analyses revealed 4 distinct protein bands with molecular masses of > 200 kDa. Of these, two were identified as α2 and β2 spectrin. The third protein band exhibited a molecular mass in the same range as spectrin (≈250 kDa), while the fourth protein band had a molecular mass much higher than spectrin (at the top of the gel), and could not be identified by mass spectrometry. To assess the reproducibility of these observations, the extraction, separation and MALDI-TOF-TOF MS/MS analysis experiments were repeated using a second set of mouse lens fiber tissue samples. The second set of experiments yielded results that were consistent with those reported for the first (Fig. 1). All of the proteins described above were identified with a confidence interval (C.I.) of > 99%.
Figure 1.
SDS-polyacrylamide gel electrophoretic separation and MALDI-TOF-TOF mass spectrometry-based identification of proteins in the triton insoluble fractions of mouse lens fibers.
To identify the major proteins of the lens fiber cell triton insoluble fraction, tissues were processed as described in Methods and separated by SDS-PAGE, using gels containing 5.5, 8 and 12% acrylamide. Gels were stained with GelCode blue and distinctly separated protein bands excised, subjected to in-gel tryptic digestion and MALDI-TOF-TOF MS analysis. Representative photographs of the GelCode stained SDS-PAGE gels are shown. The MALDI-TOF-TOF based protein identity is indicated next to the corresponding protein band on the gels.
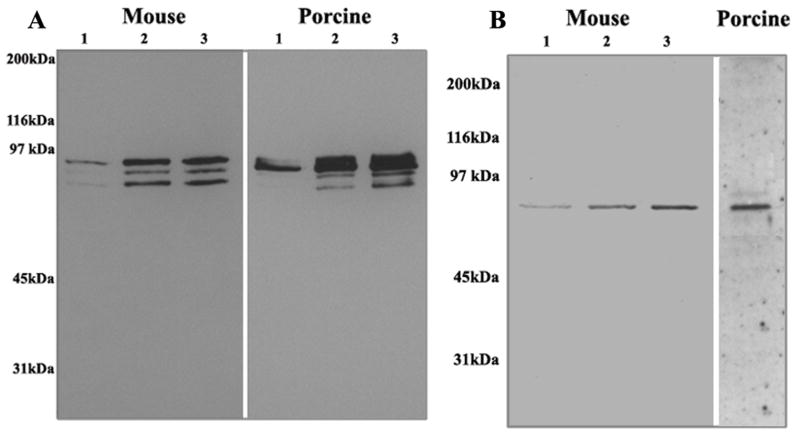
For independent confirmation of the MALDI-TOF-TOF-based identification of lens fiber cell triton insoluble proteins, immunoblotting analyses were conducted using specific polyclonal or monoclonal antibodies. Immunoblot analysis using a polyclonal phospho-Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558) antibody revealed three distinct and specific immunopositive bands representing ezrin, radixin and moesin (upper, middle, lower, respectively) in both mouse and porcine lens samples (Fig. 2A). The presence of Hsc-70 protein in the lens triton cytoskeletal fraction was confirmed in both mouse and porcine fiber cells by immunoblot analysis, with the anti-Hsc-70 antibody yielding a strong and specific immunopositive band in both mouse and porcine lens fractions (Fig. 2B). Similarly, the presence of both β- and γ-crystallins was confirmed in the mouse lens triton cytoskeletal fraction by immunoblot detection using polyclonal antibodies raised against β- and γ-crystallins (data not shown).
Figure 2.
Immunological identification of the ERM proteins and Hsc-70 in lens fiber cell triton-insoluble fractions. For an independent confirmation of the MALDI-TOF-TOF MS-based protein identification results, immunoblot analysis was carried out to identify specific proteins using polyclonal antibodies. Different amounts of lens fiber cell triton insoluble protein fractions obtained from both mouse and porcine were immnoblotted for ERM (panel A) and Hsc-70 (panel B) proteins using phospho-threonine-specific anti- ERM and anti-Hsc 70 antibodies, respectively.
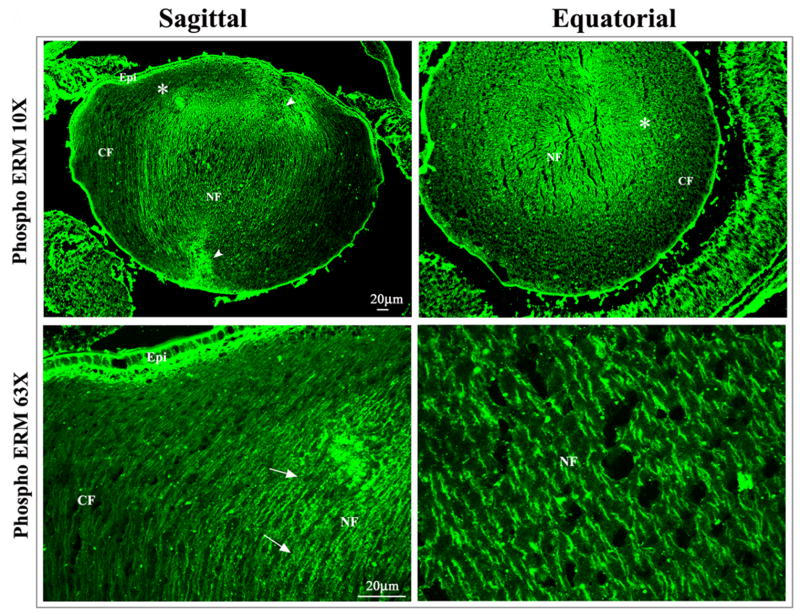
To address the potential role of ERM proteins in lens epithelial cell elongation and differentiation, we determined phospho-ERM distribution in the neonatal (P1) mouse lens based by immunofluorescence staining of sagittal and equatorial cryosections using anti-phospho ERM polyclonal antibody. In both sagittal and equatorial lens sections, the epithelium and fiber cells exhibited specific immunopositive staining for phospho-ERM (Fig. 3A). The basal and apical surface of the lens epithelium was strongly stained, while the intercellular junctions were also specifically but marginally stained. The center or nuclear region (indicated with arrows) including the suture lines (indicated with arrow heads) of the lens representing the organelle free-zone was stained much stronger than the outer or cortical regions (Fig. 3A). Phospho-ERM was noted to distribute along the lateral membrane of the lens fibers in sagittal lens sections. In the equatorial sections, phospho-ERM distribution was found to be identical to that of phalloidin-stained F-actin, co-localizing at the short sides of the hexagonal lens fiber cells (Fig. 3A, actin staining was not shown). In cultured mouse lens primary epithelial cells, phospho-ERM was localized selectively to the filopodia, lamellipodia and to the other cell surface protrusions including micovilli (Fig. 3B).
Figure 3.
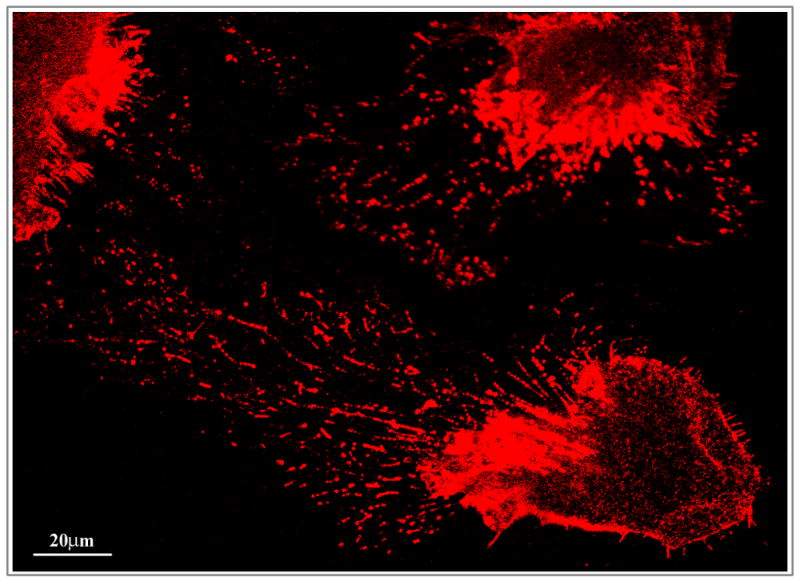
Distribution of phosphorylated ERM proteins in the neonatal mouse lens and in cultured mouse lens epithelial cells. A. To determine the distribution pattern of activated (phosphorylated) ERM proteins in the lens, P1 mouse lens cryosections (sagittal and equatorial planes) were immunostained with polyclonal antibody raised against phospho-specific ERM protein in conjunction with Alexa Fluor 488 conjugated secondary antibody. Immunofluorescence images were captured using confocal microscopy at 10x (upper panels) and 63X (lower panels) magnification. Arrows and arrowheads indicate staining at the lens fiber cell lateral membrane and lens sutures, respectively. Asterisks in upper panels depict the area that is shown at higher magnification in lower panels. Epi: Epithelium, CF: Cortical fibers and NF: Nuclear fibers. B. Distribution of phospho-ERMs in the cultured mouse lens epithelial cells. The cell apical region and cell surface protrusions including filopodia and microvilli exhibit specific localization of phospho-ERM proteins (red immunofluorescence staining).
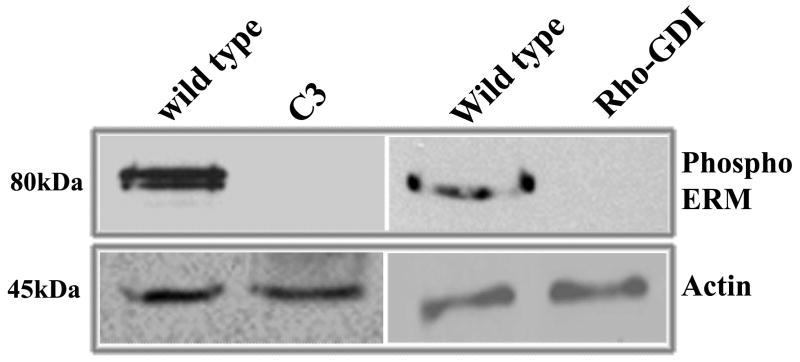
Water insoluble extracts (100,000×g pellet) derived from neonatal lenses of transgenic mice expressing the C3 exoenzyme and Rho GDIα revealed dramatically reduced levels of phospho-ERM proteins relative to lenses from their wild-type littermate controls. Four to six lenses were pooled per sample in these analyses, with two independent pooled samples exhibiting similar results (Fig. 4).
Figure 4.
Phosphorylation status of ERMs in Rho GTPase targeted transgenic lenses. Transgenic mouse lenses (one day old) expressing either C3-exoenzyme, an inactivator of RhoA, B and C, or expressing Rho GDP dissociation inhibitor (Rho GDIα), a negative regulator of Rho, Rac and Cdc42, were analyzed for changes in ERM protein phosphorylation by immunoblot analysis. The water insoluble fractions (100,000×g pellets) derived from the pooled lenses of transgenic and wild type littermates, were dissolved in urea sample buffer and equal amounts of protein from both transgenic and wild type specimens were subjected to analysis. Actin in the 800×g supernatants of the lens homogenates was immunoblotted to confirm equality of protein loading.
Discussion
Lens epithelial cell elongation and differentiation is associated with dramatic changes in cell morphology, membrane architecture, cortical cytoskeletal organization and cell-cell adhesions [2, 4]. In our previous studies, we have demonstrated a critical role for the Rho GTPases, which regulate actin cytoskeletal dynamics, in lens growth, differentiation and integrity [10, 11]. In an attempt to further our understanding of the role played by cytoskeletal reorganization in lens differentiation, this study was focused on identifying both, the major cytoskeletal components, and proteins associated with the cytoskeleton in differentiating lens fibers.
Characterization of the protein profile of fiber cell triton-insoluble fractions derived from neonatal mouse and porcine lenses has identified all the major cytoskeletal proteins including spectrin (both α and β), β-actin, vimentin, tubulin, and the lens-specific beaded filament proteins, filensin and phakinin. Importantly, in addition to these major cytoskeletal proteins, this study also demonstrates that proteins of the ERM family, which serve as cross-linkers of the actin filament network and plasma membrane, are major cytoskeleton-associated proteins in both the mouse and porcine lens. The ERM proteins, which belong to the protein 4.1 superfamily, regulate the structure and function of specific subdomains of the cell cortex and participate in a variety of cellular processes, including motility, cell adhesion, the determination of cell shape, membrane trafficking and intercellular signaling [14, 15]. These proteins are ubiquitously expressed and posses highly conserved structural domains [15]. The ERM proteins are concentrated in actin-enriched specialized plasma membrane structures and regulate membrane morphogenesis [14, 15], and share a common N-terminal domain called FERM (Four point one Ezrin, Radixin, Moesin) and a C-terminal domain that interacts with plasma membrane-associated proteins and F-actin, respectively [14]. While the presence of the ERM proteins in lens cortical complexes has recently been demonstrated [16, 17], the regulation of membrane localization and function of these proteins as it relates to lens fiber differentiation is not understood.
ERM protein function is regulated by conformational changes, and intramolecular interaction between the N- and C-terminal domains of ERM proteins is known to mask binding sites, rendering the protein dormant [14, 15, 18]. The intermolecular interactions are in turn regulated via phosphorylation of the C-terminal domain and binding of the FERM domain to phospholipids[14, 15, 18]. There is a conserved threonine residue present in all three ERM proteins, at position 567, 564, 558, in Ezrin, radixin and moesin, respectively [15, 18], which is the target for phosphorylation by Rho kinase, protein kinase C and NIK kinase [14, 15, 18, 19]. Phosphorylation at this residue relieves intramolecular interaction and produces an active protein [15]. In this study, using threonine phospho-specific ezrin (Thr567), radixin (Thr564) and moesin (Thr558) polyclonal antibody, we demonstrated that all three ERM proteins exist as phosphorylated, active forms in the triton-insoluble fraction obtained from both mouse and porcine lenses. Interestingly, phosphorylated ERMs were found to co-localize with actin filaments and exhibit a more intense distribution in the organelle-free fibers, and at the lens sutures relative to the peripheral cortical fibers. A previous study on the distribution of cell adhesion proteins also reported that the content of vinculin and paxillin, which represent other proteins associated with actin, is increased in differentiated lens fibers (organelle-free fibers), compared to early differentiating fiber cells [6]. In addition to the loss of organelles, differentiated fibers exhibit increased lateral membrane reorganization and formation of extensive ball and socket interdigitations [4, 20]. Based on these prior observations, it is likely that the presence of increased levels of phospho-ERM in the organelle-free differentiated lens fibers is related to membrane remodeling and formation of membrane interdigitations, and fiber cell packing.
In preliminary experiments, we have also observed increased ERM protein phosphorylation in lens epithelial cells treated with serum and different growth factors (Maddala and Rao unpublished). Growth factors play a fundamental role in lens epithelial cell proliferation and differentiation [21], and have been demonstrated to activate Rho GTPases in lens epithelial cells [13]. ERM proteins regulate Rho GTPase membrane targeting and activity by recruiting regulators of Rho GTPases such as Rho GDI (Rho GDP dissociation inhibitor) and RhoGEFs (Rho guanine nucleotide exchange factor) [15, 22, 23]. On the other hand, Rho kinase also regulates ERM protein phosphorylation [24]. Specifically targeting Rho GTPase activity in the developing lens via overexpression of C3-exoenzyme or RhoGDIα, was found to result in defects in lens integrity, cytoskeletal organization and differentiation [10, 11], as well as markedly reduced levels of phospho-ERM in these transgenic mouse models (Fig. 4). Although it is not presently clear that a cause and effect relationship exists between the decreased levels of phospho-ERM proteins and inactivation of Rho GTPases in the lenses of these transgenic models, the fact that ERMs and Rho GTPases are capable of mutual regulatory interactions suggests their importance in lens differentiation and function.
Acknowledgments
We thank Dr. Sam Zigler for his critical comments on the manuscript. This work was supported by a grant from the National Institutes of Health (R01EY12201) and Research to Prevent Blindness to P.V.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13(Pt 3b):425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- 2.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 3.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 4.Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–1410. [PubMed] [Google Scholar]
- 5.Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin Cell Dev Biol. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–734. [PubMed] [Google Scholar]
- 7.Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Dev Dyn. 2000;217:257–270. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Ramaekers FC, Boomkens TR, Bloemendal H. Cytoskeletal and contractile structures in bovine lens cell differentiation. Exp Cell Res. 1981;135:454–461. doi: 10.1016/0014-4827(81)90190-7. [DOI] [PubMed] [Google Scholar]
- 9.Graw J. Congenital hereditary cataracts. Int J Dev Biol. 2004;48:1031–1044. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- 10.Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84:679–692. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- 11.Maddala R, Reneker LW, Pendurthi B, Rao PV. Rho GDP Dissociation Inhibitor-Mediated Disruption of Rho GTPase Activity Impairs Lens Fiber Cell Migration, Elongation and Survival. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.12.039. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–336. [PubMed] [Google Scholar]
- 14.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 15.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem. 1999;274:34507–34510. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 16.Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci. 2003;116:4985–4995. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- 17.Bagchi M, Katar M, Lo WK, Yost R, Hill C, Maisel H. ERM proteins of the lens. J Cell Biochem. 2004;92:626–630. doi: 10.1002/jcb.20062. [DOI] [PubMed] [Google Scholar]
- 18.Gautreau A, Louvard D, Arpin M. ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol. 2002;14:104–109. doi: 10.1016/s0955-0674(01)00300-3. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, Wright JH, Barber DL. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci U S A. 2006;103:13391–13396. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blankenship T, Bradshaw L, Shibata B, Fitzgerald P. Structural specializations emerging late in mouse lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2007;48:3269–3276. doi: 10.1167/iovs.07-0109. [DOI] [PubMed] [Google Scholar]
- 21.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]