Fig. 6.
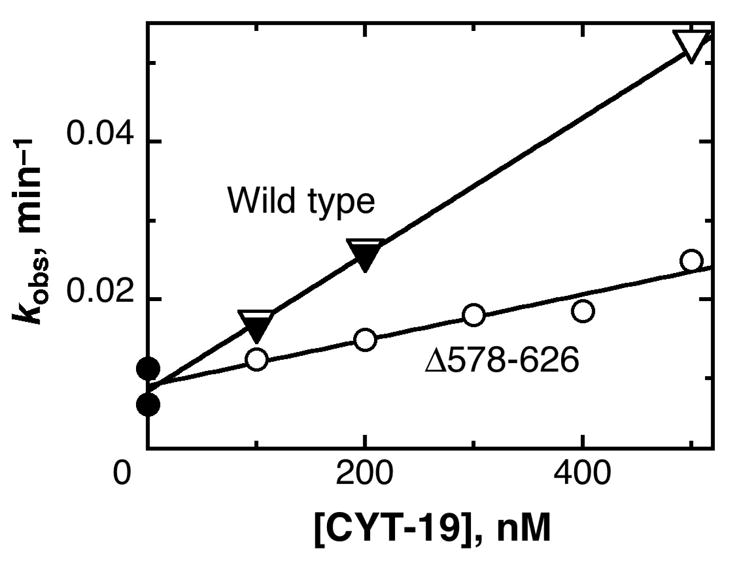
Acceleration by CYT-19 of misfolded Tetrahymena ribozyme re-folding to the native state. The progress of re-folding was followed by the onset of substrate cleavage activity by the ribozyme in the absence of CYT-19 (λ) or in the presence of various concentrations of Δ578–626 (○) or wild-type CYT-19 (performed side-by side) (τ). An equivalent experiment for the wild-type CYT-19 protein has been published previously, and these data are included for comparison (∇). The dependences of re-folding rate constant on protein concentration gave kcat/KM values of 8.6 (± 0.4) × 104 M−1 min−1 and 2.9 (± 0.4) × 104 M−1 for the wild-type and Δ578–626 proteins, respectively.
