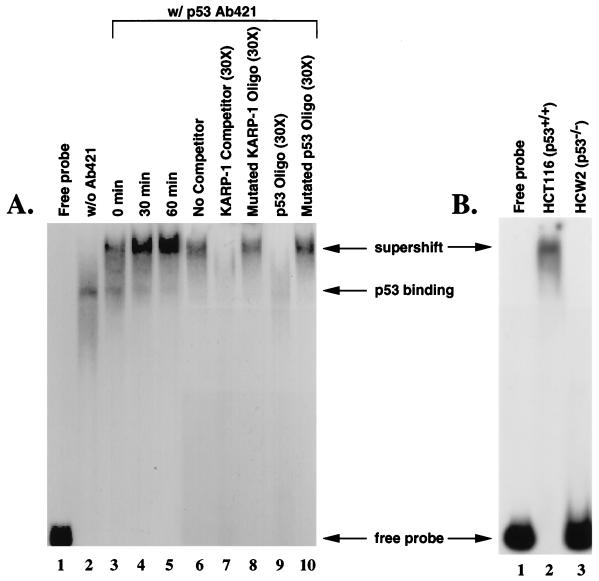
Figure 2.
p53 can bind to the KARP-1 p53 response element. (A) Whole cell extracts were prepared from HCT116 (p53+/+) cells combined with a 183 bp probe containing the putative KARP-1 p53 response element in the absence (lanes 1 and 2) or presence (lanes 3–10) of the p53-specific antibody, pAb421, and subjected to a gel retardation assay: the probe incubated without cellular extract (lane 1); extracts from unirradiated cells (lanes 2 and 6); extracts from x-irradiated (15 Gy) cells prepared 0 min (lane 3), 30 min (lane 4), or 60 min (lane 5) postirradiation; addition of 30-fold excess cold 183 bp probe (lane 7); addition of 30-fold excess of a double-stranded oligonucleotide containing mutations in the KARP-1 p53 binding site (lane 8); addition of 30-fold excess of a double-stranded oligonucleotide containing a dimer of the p21 promoter consensus p53 binding site (lane 9); addition of 30-fold excess of a double-stranded oligonucleotide containing mutations in the p21 promoter consensus p53 binding site (lane 10). (B) Binding to the KARP-1 probe is not observed in p53-defective cell lines. Whole cell extracts were prepared from HCT116 (p53+/+; lane 2) and HCW-2 (p53−/−; lane 3) cells combined with a 183 bp probe containing the putative KARP-1 p53 response element in the presence of the p53-specific antibody, pAb421, and subjected to a gel retardation assay.