Scheme 1.
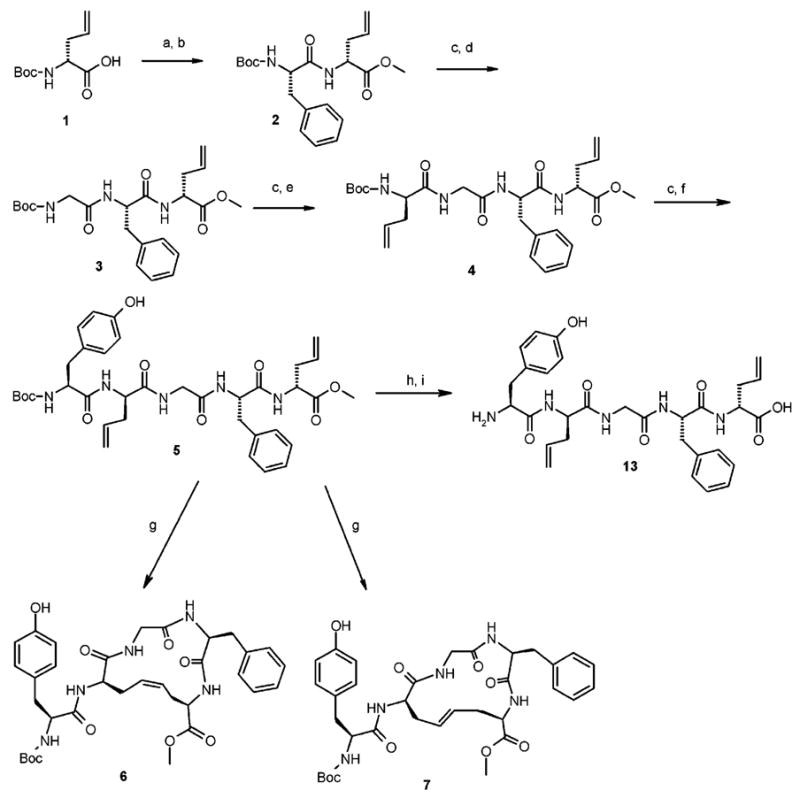
Synthesis of Fully Protected H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH Analogues 5, 6, and 7 and Linear Deprotected Product 13a
a Reagents and conditions: (a) SOCl2/MeOH 10 min at 0 °C, then 6 h at rt; (b) Boc-Phe-OH/EDC/HOBT•H2O/DMF/NMM, 16 h at rt; (c) TFA/CH2Cl2 (1:2) 1 h, rt; (d) Boc-Gly-OH/EDC/HOBT•H2O/NMM/DMF, 16 h at rt; (e) Boc-D-Allyl-Gly-OH/EDC/HOBT•H2O/NMM/DMF, 16 h at rt; (f) Boc-Tyr-OH/EDC/HOBT•H2O/NMM/DMF, 16 h at rt; (g) Grubbs’ catalyst second generation (20%)/CH2Cl2 at rt; (h) TFA/CH2Cl2 (1:2) 1 h at rt; (i) NaOH 1 N 6 equiv/MeOH, 4 h at rt.
