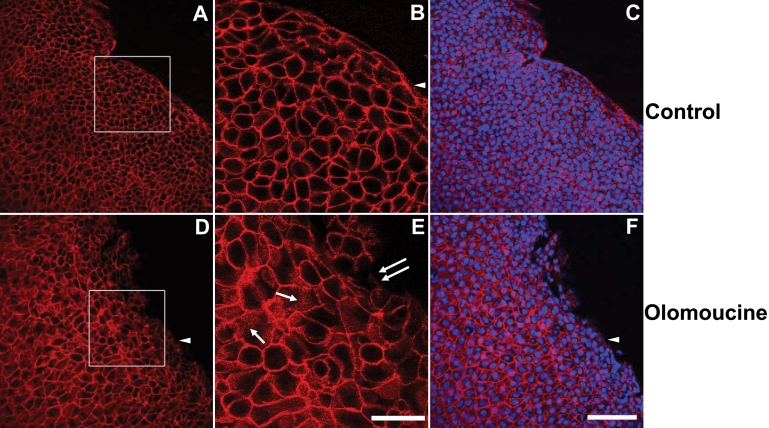
Figure 2.
Olomoucine treatment does not disrupt the integrity of the epithelial cell sheet. A: The immunofluorescence of E-cadherin in epithelial cells of corneal whole mounts of control animals shows tightly packed epithelial cells with a smooth, advancing cell front. Cells were compact and cell density was high especially along the wound edge. B: Higher magnification of the boxed area shown in panel A shows that E-cadherin immunofluorescence in control corneas was confined almost entirely to cell-to-cell boundaries. Punctate E-cadherin staining was seen at the migrating front on the edge lacking cell-to-cell contacts (arrowhead). C: Superimposition of E-cadherin immunostaining and DAPI-staining of nuclei confirms that E-cadherin is located at cell-to-cell boundaries and that all cells express E-cadherin. D: In olomoucine-treated corneas, cell density was appreciably lower and the migrating front was irregular. Cell-to-cell junctions appeared to be intact except for a few cells at the wound edge. Immunostaining of E-cadherin was weak or undetectable at the migrating front on the edge lacking cell-to-cell contacts (arrowhead). E: Higher magnification of the boxed area shown in panel D demonstrates punctate intracellular immunostaining for E-cadherin in many cells (single arrows). E-cadherin localization at cell-to-cell junctions was disrupted in a few cells along the wound edge (double arrows). F: Superimposition of E-cadherin immunofluorescence and DAPI-staining of nuclei confirms that E-cadherin is located at cell-to-cell boundaries and demonstrates that all cells express E-cadherin. Scale bar=100 μM in A,C,D, F; 40 μM in B,E.