Fig. 2.
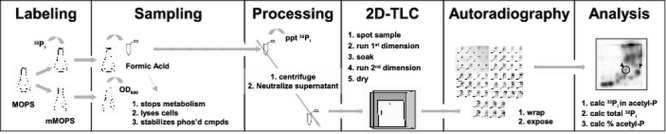
Schematic of the procedure. An overnight culture is grown in MOPs media supplemented with the desired carbon source. In the morning, two parallel mMOPS cultures are prepared: one "hot" and one "cold". The "hot" culture is supplemented with [32Pi] and is used to continuously label the cells. The "cold" culture is used to monitor growth in terms of optical density at 600 nm (OD600) and is prepared such that the surface:volume ratio remains identical to that of the "hot" culture throughout the entire sampling process. At regular intervals, a 100 μl sample is removed from the"hot" culture and immediately dispensed into 10 μl of 11 N formic acid. The formic acid stops metabolism, lyses the cells, and stabilizes phosphorylated compounds. The sample is then processed, precipitating the unincorporated [32Pi], centrifuging the precipitant, and neutralizing the supernatant fluid. 10 μl of the supernatant is applied to one corner of a TLC plate and permitted to dry. The plate is then rinsed with methanol, thoroughly dried, and immersed in the first dimension solvent. After development in the first dimension, the plate is soaked in methanol, allowed to thoroughly dry, turned 90 degrees, and immersed in the second dimension solvent. After development in the second dimension, the plate is removed, soaked in methanol and allowed to thoroughly dry. The plate is wrapped and exposed to a phosphorimager screen overnight. The plate is scanned and the percentage of acetyl-P is calculated by dividing the amount of signal corresponding to acetyl-P by the total signal.
