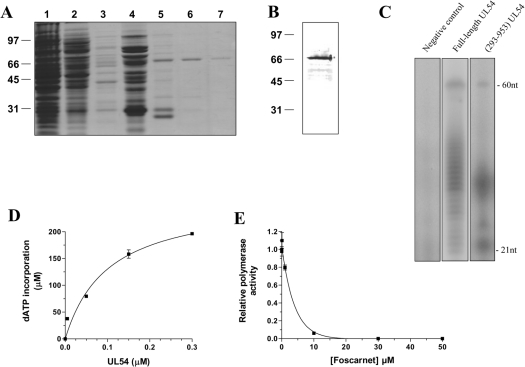
Figure 2. Purification and enzymatic activity of the purified cytomegalovirus DNA polymerase.
(A) The peptide compositions of various purification fractions were analysed by SDS/PAGE. The gels were fixed and stained with Coomassie Blue dye. Lane 1, Ni-NTA–agarose flow-through; lane 2, Ni-NTA–agarose wash; lane 3, 50 mM imidazole eluate; lane 4, 100 mM imidazole eluate; lane 5, 200 mM imidazole eluate; lane 6, 500 mM imidazole eluate; lane 7, 1000 mM imidazole eluate. The positions and sizes (in kDa) of the size markers are indicated on the left. (B) The purified cytomegalovirus protein (500 mM imidazole fraction) was analysed by immunoblotting using a monospecific anti-His antibody. (C) Polymerization assays performed with a heteropolymeric primer/template DNA substrate (P20/T60). The reaction mixtures (50 μl) containing 20 mM Tris/HCl (pH 7.5), 10 mM MgCl2, 50 mM NaCl, 5 μM of primer/template DNA substrate, 0.2 mM [α-32P]ATP and 500 mM of dGTP, dCTP and dTTP were incubated for 30 min at 37 °C in the presence of 0.6 μM of either the full-length protein expressed in a coupled transcription/translation system (lane 2) or recombinant enzyme (lane 3). A negative control (vector alone) synthesized with the coupled transcription/translation system was also used in the assay (lane 1). The reaction products were analysed by electrophoresis through a 10% polyacrylamide gel containing 8 M urea. An autoradiogram of the gel is shown, and the positions of the size markers are also indicated. (D) Increasing amounts of enzyme were added to the heteropolymeric primer/template DNA substrate (P20/T60). The reaction mixtures containing 5 μM of primer/template DNA substrate, 0.6 μM of enzyme, 1 mM [α-32P]ATP and 1 mM of dGTP, dCTP and dTTP were incubated for 30 min at 37 °C. The reaction products were analysed by liquid-scintillation counting following the precipitation of the DNA reaction products. (E) Polymerization assays were performed in the presence of increasing concentrations of foscarnet, and the incorporation of radiolabelled ATP was quantified by liquid-scintillation counting.