Summary
Most drugs of abuse increase dopamine (DA) in nucleus accumbens (Acb). However, the effects of anabolic androgenic steroids (AAS) on Acb DA have not been examined. We determined the effects of subcutaneous (sc) testosterone (T) on Acb DA in male hamsters. The effects of sc amphetamine were also examined for comparison. In addition, Acb DA was evaluated during intracerebroventricular (ICV) T infusion, designed to mimic T intake during ICV T self-administration in drug-naïve and drug-preexposed animals. Acb DA was measured using in vivo microdialysis and HPLC-EC. T (7.5 or 37.5 mg/kg), amphetamine (1 or 5 mg/kg), or vehicle was injected sc and Acb DA monitored for 4 hrs. In the ICV experiment, T (1 or 2 μg/infusion) or vehicle was infused ICV every 6 min for 4 hrs and Acb DA monitored. ICV T preexposure was accomplished by repeating the same ICV T infusion (1 μg/infusion) daily for 14 days, and T infusion was accompanied by microdialysis on 15th day. Neither sc nor ICV T administration increased Acb DA. At high dose (2 μg/infusion), ICV T decreased Acb DA. Likewise, daily ICV infusion of T for 15 days did not alter Acb DA. In contrast, sc amphetamine significantly increased Acb DA at both doses. Therefore, unlike many drugs of abuse, AAS does not increase Acb DA levels. The reduction in DA at high T doses is likely due to autonomic depressant effects of AAS. We suggest that AAS act via mechanism distinct from those of stimulants, but may share neural substrates with other drugs of abuse.
Keywords: anabolic androgenic steroid, testosterone, amphetamine, hamster, nucleus accumbens, dopamine, microdialysis, HPLC-EC, intracerebroventricular, subcutaneous
Androgenic anabolic steroid (AAS) use is widespread among athletes and non-athletes (Yesalis et al., 1993). Physical (Leshner, 2000) and psychological (Brower, 2002; Pope and Katz, 1994) effects of AAS use are of significant concern from a public health perspective. Brower (2002) has recently suggested that most AAS users initiate use for the anabolic properties, but many subsequently develop physical and psychological dependence. However, the addictive potential of AAS has received little attention so far. The results of studies using animal models of drug abuse indicate that AAS are reinforcing. For example, AAS induces conditioned place preference (CPP) in rats (Packard et al., 1997) and mice (Arnedo et al., 2000). In addition, AAS are self-administered through various routes in rats (Sato et al., 2006; Wood et al., 2004) and hamsters (Ballard and Wood, 2005; DiMeo and Wood, 2006b; Frye et al., 2007; Johnson and Wood, 2001; Peters and Wood, 2005; Wood, 2002). However, it is not known how AAS affect neural circuitry underlying the reinforcing effects of other drugs of abuse.
The mesolimbic dopamine (DA) system, the DAergic projection from the ventral tegmental area (VTA) to the nucleus accumbens (Acb), is a major substrate for drugs of abuse (Berridge and Robinson, 1998; Koob and Nestler, 1997). Selective lesion of DAergic fibers disrupts self-administration of stimulants (Roberts et al., 1977). DA antagonists also have been shown to attenuate the reinforcing effects of cocaine during self-administration (Caine & Koob, 1994). In addition, most commonly-abused drugs are known to increase DA levels in Acb, including stimulants (Di Chiara and Imperato, 1988), opiates (Di Chiara and Imperato, 1988), ethanol (EtOH, Di Chiara and Imperato, 1985), and nicotine (Imperato et al., 1986).
There is some evidence implicating a role for the mesolimbic DA system in AAS abuse. For example, AAS induces CPP when injected into Acb (Packard et al., 1997), an effect blocked by the DA antagonist α-flupenthixol (Packard et al., 1998). Furthermore, acute intracerebroventricular (ICV) administration of AAS induces c-Fos expression in the VTA (Dimeo and Wood, 2006a). Based on these data, we have hypothesized that the reinforcing effects of AAS are mediated by the mesolimbic DA system. If AAS utilize the same neural substrates as other drugs of abuse, then AAS should also increase DA in the Acb. The current study was designed to examine Acb DA release in response to AAS administration. Acb DA release was measured in hamsters, using in vivo microdialysis with high performance liquid chromatography with electrochemical detection (HPLC-EC). We examined the effects of acute systemic administration of testosterone (T) on Acb DA release. As a control, we also examined the effects of acute amphetamine administration on Acb DA. Furthermore, we examined the effects of ICV T infusions designed to mimic drug intake during self-administration. Finally, we tested the effects of ICV T following repeated (15 day) ICV T administration, in order to control for possible interference from the autonomic depressant effects of T.
Materials and Methods
Animals
Adult male Syrian hamsters (120–160g BW) were obtained from Charles River Laboratories (Wilmington, MA). Hamsters were housed individually under a reversed long-day photoperiod (14L: 10D) with lights off at 9 AM. Food and water were available ad libitum. All tests were conducted during the dark phase of their light cycle.
Experimental Design
In the first experiment, we examined the effects of an acute injection of systemic T on Acb DA release. Following baseline sample collection, hamsters received a subcutaneous injection of 7.5 mg/kg T (n = 5), 37.5 mg/kg T (n = 5), or vehicle (n = 5). Acb DA levels were monitored for 4 hrs following the injection. The lower dose (7.5 mg/kg) has been used previously in rats and mice to test the effects of androgens on seizure activity (Frye and Reed, 1998), anxiety (Rojas-Ortiz et al., 2006), aggression (Martinez-Sanchis et al., 1998), and social behavior (Barreto-Estrada et al., 2004). Furthermore, 7.5 mg/kg testosterone is comparable to doses tested in human volunteers. According to the National Center for Health Statistics (NHANES, 2007), the average American man weighs 86 kg. Thus, a typical human dose of 600 mg testosterone (Bhasin et al., 1996; Kouri et al., 1995; Tricker et al., 1996) is equivalent to 7 mg/kg.
The Acb DA responses to drugs of abuse have not been previously examined in Syrian hamsters. Therefore, we examined Acb DA response to amphetamine in hamsters as a control. Hamsters were subcutaneously injected with either 1 or 5 mg/kg amphetamine, and Acb DA was monitored for 4 hrs. These doses of amphetamine are known to induce robust increases in Acb DA in rats (Birgner et al., 2007; Di Chiara and Imperato, 1988).
In the second set of experiments, Acb DA levels were examined using a drug infusion paradigm similar to ICV self-administration. Following baseline sample collection, T was infused through a modified microdialysis probe inserted into the lateral ventricle. Drug-naïve animals received vehicle (n = 5), 1 μg/infusion T (40 μg total; n = 6), or 2 μg/infusion T (80 μg total; n = 4) every 6 min over 4 hrs. Solutions were delivered as 1 μl infusions every 6 min (40 μl/4hrs), using a programmable syringe pump (BS-8000, Braintree Scientific, Braintree, MA). Acb DA was monitored throughout the 4 hr infusion. Forty μg (1 μg/infusion) T was designed to approximate a heavy dose of T during ICV T self-administration, while 80 μg (2 μg/infusion) T is a maximal dose. We have previously used this method to examine ICV T-induced c-Fos expression (Dimeo and Wood, 2006a) and physiologic effects of T (Peters and Wood, 2005).
As we have previously demonstrated, T exerts autonomic depressant effects when administered ICV (Peters and Wood, 2005). It is possible that any influence of T on Acb DA may be masked by its autonomic depressant effects. In order to control for this possibility, we infused 1 μg/infusion T (n = 5) ICV daily for 14 days as described above. This duration is sufficient for hamsters to develop tolerance to autonomic depressant effects of T (Peters and Wood, 2005). On 15th day, T infusion was accompanied by microdialysis sampling.
Surgery
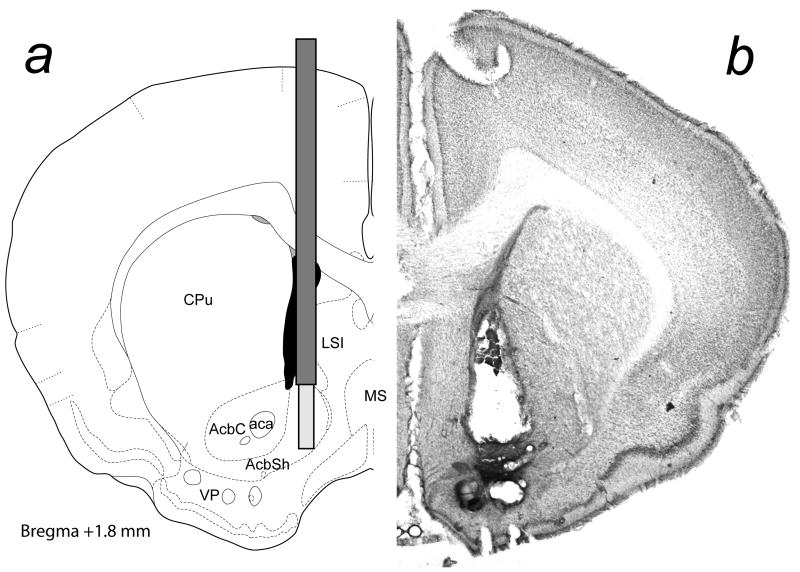
Surgical procedures were carried out under aseptic conditions according to “Principles of laboratory animal care” (NIH publication NO. 86-23, revised 1985). Hamsters were anesthetized with sodium pentobarbital (80 mg/kg) and secured in a Kopf stereotaxic apparatus with lambda and bregma in the same horizontal plane. A microdialysis guide cannula (CMA/12, CMA, N. Chelmsford, MA) was lowered to 1 mm above Acb. Stereotaxic coordinates were: AP: +3.3 mm, ML: +1.1 mm, DV: − 6.0 mm from bregma, (Fig. 1a, Morin and Wood, 2001). For ICV infusion, another guide cannula was implanted into the lateral ventricle (AP: +0.8 mm, ML, −1.2 mm, DV: −5.0 mm from bregma). Dummy cannulae were inserted to prevent the entry of foreign material. The cannula assemblies were secured to the skull with stainless steel screws and dental acrylic. After surgery, males were allowed to recover for at least 5 days before microdialysis sampling.
Fig. 1.
The location of a) microdialysis probe, and b) the photomicrograph of a representative microdialysis probe placement. Coordinates are based on a stereotaxic atlas of the golden hamster brain (Morin and Wood, 2001).
Drugs
Testosterone (Steraloids, Newport, RI) was dissolved in an aqueous vehicle of 13% 2-hydroxyorpyl-β-cyclodextrin (βCD, RBI, Natick, MA). Glass was used for preparation and delivery of testosterone since steroids readily adsorb to plastic (Bruning et al., 1981). D-amphetamine (Sigma-Aldrich, St. Louis, MO) was dissolved in the same vehicle.
Microdialysis and DA analysis
Testing was performed in a 30 cm2 glass aquarium. To obtain a stable baseline of DA release, the microdialysis probe (CMA/12, 1 mm dialysis membrane) was inserted at least 12 hrs before testing, and perfused with artificial cerebrospinal fluid (aCSF, pH 7.4, Harvard Apparatus, Holliston, MA). The flow of aCSF (1.5 μl/min) began immediately after probe insertion, and was controlled by a Harvard Model 11 syringe infusion pump. A 15–22.5 μl dialysate was collected every 10–15 min. Baseline samples were collected for at least 50 min before drug infusion or injection, and sampling continued for the next 4 hrs. Dialysates were collected into a centrifuge tube and frozen until analysis.
Dialysates were loaded via a Rheodyne injector valve (Model #9125, Rheodyne Inc., Cotati, CA), isolated using a reverse-phase column (ESA MD-150, Chelmsford, MA) and quantified using an ESA Coulochem II detector (ESA model 5200) comprising a guard cell (+350 mV, ESA model 5020) and an analytical cell (ESA model 5014B) with two electrodes in series. The potentials of the electrodes were set at −125 mV and +125 mV to detect DA at the second electrode. Flow rate of the mobile phase (ESA MD-TM) was 0.6 ml/min using an ESA pump (Model 582). Data were collected using PowerChrom software (AD instruments, Mountain View, CA) linked to a Macintosh computer. The detection threshold for DA was ≥ 100 fg at 3:1 SNR. Due to the low levels of DA observed in some groups, the system was optimized for detection of DA. As a result, DA metabolites were often out of detection range, thus not analyzed in the current study.
Statistical Analysis
Basal DA release was determined from the average of the last 3 baseline samples. Subsequent samples were expressed as a percentage of the mean baseline value. The percent changes from the baseline were calculated for each sample, and consecutive samples were pooled to produce percent baseline values for 30 min samples. Each treatment condition was analyzed with 1-way repeated measures ANOVA, followed by Tukey’s multiple-comparions test when appropriate. In all analyses, p < 0.05 was considered significant.
Histology
At the end of the experiment, each male was deeply anesthetized with sodium pentobarbital and perfused through the aorta with 150 ml of 0.1 M phosphate-buffered saline containing 0.1 % sodium nitrite for vasodilation, followed by 250 ml of 0.1 M sodium phosphate buffer containing 4 % paraformaldehyde. Brains were removed and post-fixed in the same fixative for 1 h at room temperature and then cryoprotected overnight in buffer with 20 % sucrose at 4°C. Probe placement was verified histologically in 60 μm coronal brain sections stained with cresyl violet. The placement of a representative microdialysis probe is shown in Fig. 1b.
Results
A bolus subcutaneous testosterone injection
The effects of an acute sc injection of T (7.5 and 37.5 mg/kg) or βCD vehicle on Acb DA levels are shown in Fig. 2. At a dose (7.5 mg/kg) known to induce CPP in rats, the sc injection of T did not significantly change DA levels (F8, 32 = 0.67, ns). Even at a much higher dose (37.5 mg/kg), sc T did not significantly alter DA levels (F8, 32 = 1.32, ns). Likewise, animals injected with βCD did not show any changes in Acb DA levels (F8, 32 = 0.80, ns). No apparent behavioral effect was observed in any group.
Fig. 2.
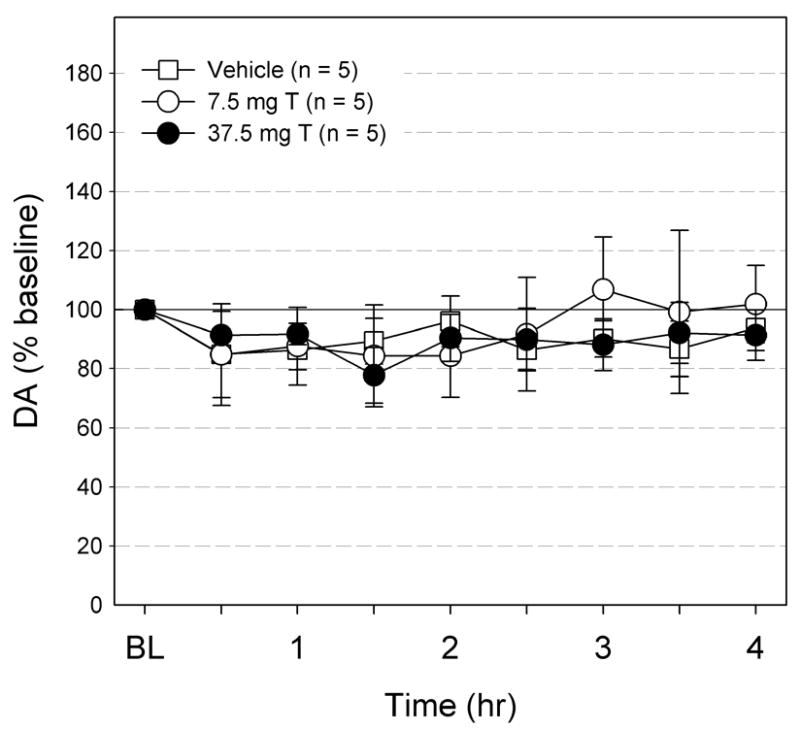
The effects of subcutaneous injection of vehicle (n =5), 7.5 mg/kg T, and 37.5 mg/kg T on Acb DA levels. The DA levels are expressed as % baseline ± S.E.M. The DA levels did not change with any of the treatments.
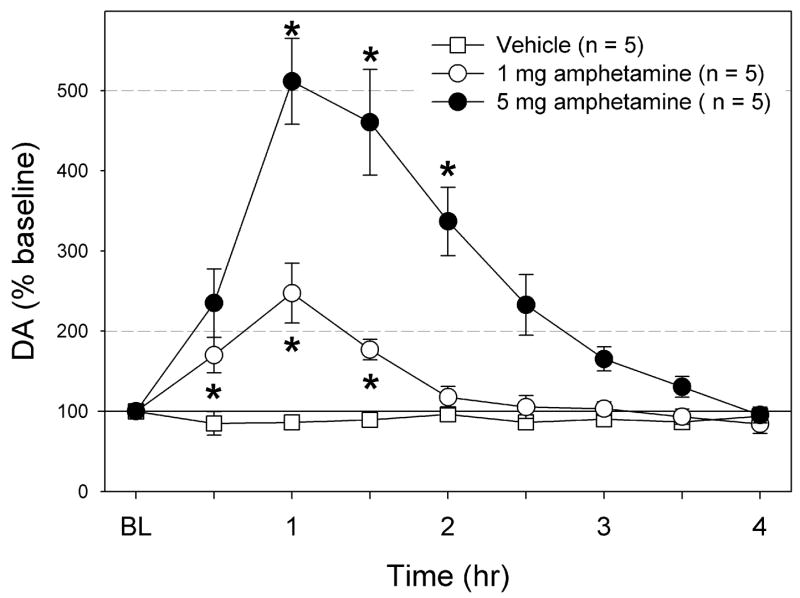
A bolus subcutaneous amphetamine injection
The effects of an acute sc injection of amphetamine are shown in Fig. 3. Amphetamine dose-dependently increased Acb DA levels. High dose amphetamine (5 mg) induced a significant DA increase up to 500 % of baseline levels, peaking at 1h after injection. At this dose, all animals exhibited increased locomotion and stereotypy. The DA increase followed a similar pattern at 1 mg/kg, but the peak level was lower: 250 % of baseline. At this dose, most animals showed little behavioral effects of amphetamine.
Fig. 3.
The effects of subcutaneous injection of vehicle (n =5), 1 mg/kg amphetamine, and 5 mg/kg amphetamine on Acb DA levels. The DA levels are expressed as % baseline ± S.E.M. The DA levels did not change with vehicle, but significantly increased with both doses of amphetamine. * Significantly different from baseline.
ICV testosterone infusion in drug-naïve animals
Fig. 4 shows the effects of ICV infusions of the βCD vehicle, low dose (1 μg/infusion) T, and high dose (2 μg/infusion) T on Acb DA in drug-naïve animals. In the animals receiving vehicle, the extracellular DA levels declined slightly, but non-significantly during the 4 hr infusion (F8, 32 = 0.77, ns). Similarly, with the low dose T (1 μg/infusion), the extracellular DA levels showed a slight decline in the first 30 min, but did not decline further. Instead, DA levels remained at 70 to 80 % of baseline (F8, 40 = 1.39, ns). In the animals receiving 2 μg/infusion T, Acb DA levels significantly decreased from baseline (F8, 24 = 15.80, p < 0.0001). The DA level decreased gradually during the first 2 hrs of infusion, reaching significance by 1hr. For the last 2 hrs of infusion, DA remained at approximately 30 % of baseline.
Fig. 4.
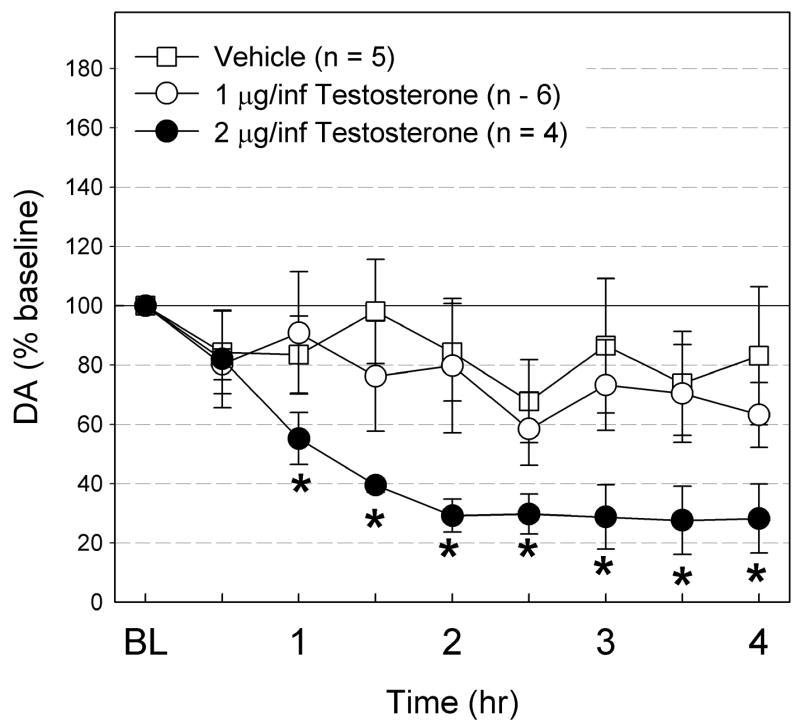
The effects of ICV infusion of vehicle (n = 5), 1 μg/infusion T (40 μg total, n = 6), and 2 μg/infusion T (80 μg total, n = 4) on Acb DA levels. The DA levels are expressed as % baseline ± S.E.M. The DA levels did not change with vehicle or T at 1 μg/infusion, but significantly declined with T at 2 μg/infusion. * Significantly different from baseline.
ICV testosterone infusion following 15-day daily ICV T infusion
Fig. 5 shows the effect of ICV T infusion on Acb DA levels following 14 days of daily 4 hr T-infusion. Similar to acute T infusion of the same dose (1 μg/infusion), ICV T infusion failed to alter Acb DA levels (F8, 32 = 0.83, ns), even after 14 days of daily ICV T infusions. The DA levels were unchanged from the baseline level for the duration of the test.
Fig. 5.
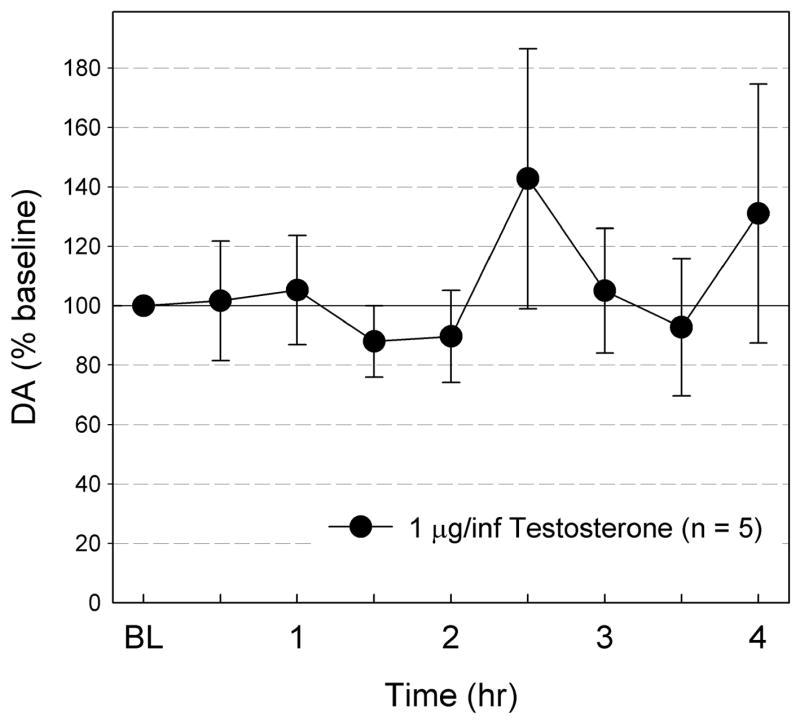
The effects of ICV T (40 μg, n = 5) infusion on Acb DA levels following 14 days of repeated ICV T infusion. The DA levels are expressed as % baseline ± S.E.M. The DA levels did not significantly change from baseline.
Discussion
Exogenous testosterone has little influence on Acb DA levels
The results of the current study suggest that testosterone does not stimulate Acb DA release at physiologically- and behaviorally-relevant doses. A bolus injection of systemic 7.5 or 37.5 mg/kg T failed to induce Acb DA increase. No sign of stereotypy or increased locomotion was apparent at either dose. The lower dose (7.5 mg/kg) is sufficient to reduce seizure activity (Frye and Reed, 1998), and to enhance aggressive behavior (Martinez-Sanchis et al., 1998) and social conflict (Barreto-Estrada et al., 2004). Furthermore, the high dose sc T (37.5 mg/kg) is much higher than those shown to have significant physiological and behavioral effects. The lack of DA release at either dose suggests subcutaneously administered testosterone does not induce Acb DA release. In contrast, subcutaneous amphetamine dose-dependently increased DA release in hamster Acb. The magnitude of amphetamine-stimulated DA increase appears to be somewhat lower in hamsters than in rats (Di Chiara and Imperato, 1988). We observed approximately 250% increase in Acb DA with 1 mg/kg amphetamine, while >800% increase has been reported in rats (Di Chiara and Imperato, 1988). The amphetamine-induced Acb DA release demonstrates that hamster Acb is similar to that in rats not only anatomically (Johnson and Wood, 1999), but functionally as well.
Most of our T self-administration studies have utilized ICV as route of administration (Wood et al., 2004, for example). Therefore, we examined Acb DA changes induced by ICV T infusion. We used a dose slightly higher than average T intake during self-administration (27 μg/4 hrs (Wood et al., 2004). We also tested 80 μg T, a dose much higher than typically self-administered. In both cases, Acb DA decreased, and animals appeared to be heavily sedated at the end of the 4 hr session. We have previously demonstrated that 40 μg T ICV produces significant depression of locomotion, respiration, and body temperature (Peters and Wood, 2005). Therefore, we suspect the observed Acb DA decrease was a byproduct of autonomic depressant effects of T, rather than direct effects on the mesolimbic DA system. In these drug naive animals, it is possible that autonomic depressant effects of T have masked any DA increase. Since hamsters develop tolerance to autonomic depressant effects of T within 15 days (Peters and Wood, 2005), we infused 1 μg/infusion T daily for 14 days, and examined the DA response on the 15th day. ICV T still failed to stimulate Acb DA release, further supporting the lack stimulant-like effects of T on Acb DA. Furthermore, the profound sedation with 80 μg ICV T suggests that higher doses cannot be tested. Therefore, it is highly unlikely that the doses used in this study were insufficient to induce Acb DA increase.
Currently, the pharmacological mechanism of AAS reinforcement is unknown. There are numerous AAS available, each with slightly different pharmacological profile. Furthermore, AAS are administered through various routes using various vehicles, thus pharmacokinetics of AAS differ significantly. AAS may even exert opposing effects, via their estrogenic and androgenic metabolites. For example, the facilitative effects of estrogens on Acb DA (Becker and Rudick, 1999) may have been masked by inhibitory effects elsewhere in the brain. Nonetheless, the results of this study have clinical relevance, because T is the most prevalent AAS (WorldAnti-DopingAgency, 2006).
The importance of the mesolimbic DA system in drug abuse is well-established (Berridge and Robinson, 1998; Koob and Nestler, 1997). Most drugs of abuse are known to increase Acb DA (Di Chiara and Imperato, 1988). Since AAS are also recognized as drugs of abuse (DSM-IV-TR, 2000), we expected to see an increase in Acb DA. Behavioral and neurochemical evidence also suggested involvement of the mesolimbic DA system in AAS abuse. For example, T-induced CPP is blocked by a DA antagonist in Acb (Packard et al., 1998). In addition, chronic AAS-treatment is known to alter monoaminergic activity in several brain structures, including Acb (Kindlundh et al., 2004; Thiblin et al., 1999). Thus, the lack of Acb DA increase with both ICV and sc T administration was unexpected. However, Birgner and colleagues (2007) have recently demonstrated an attenuated Acb DA response to amphetamine in rats treated with chronic AAS. In light of the results of these data, we suspect that the relationship between the mesolimbic DA system and AAS abuse may be indirect. In fact, the relative paucity of nuclear androgen receptors in Acb or the VTA is well-documented (Kritzer, 1997; Simerly et al., 1990; Wood and Newman, 1995). Therefore, the influence of AAS on the mesolimbic DA system is likely to be mediated by androgen-sensitive afferents and/or non-classical androgen receptors (Simoncini and Genazzani, 2003).
AAS is another drug of abuse with DA-independent effects
Based on this and previous studies, it is clear that AAS do not resemble stimulants. Instead, AAS appear to be another class of non-stimulant drugs, with at least some of their behavioral effects mediated by DA-independent pathways. Self-administration of opiates (Pettit et al., 1984), ethanol (EtOH; Rassnick et al., 1993), and phencyclidine (Carlezon and Wise, 1996) are not dependent on mesolimbic DA. In particular, although benzodiazepines reduce Acb DA (Finlay et al., 1992; Invernizzi et al., 1991; Zetterstrom and Fillenz, 1990), they are nonetheless self-administered (Naruse and Asami, 1987; Szostak et al., 1987) and produce CPP (File, 1986; Spyraki and Fibiger, 1988; Spyraki et al., 1985). Likewise, EtOH-induced CPP is not DA-dependent (Cunningham et al., 1992; Risinger et al., 1992). Even drug-induced locomotion, which is closely linked to Acb DA, may not be always dependent on Acb DA for opiates (Kalivas et al., 1983; Pert and Sivit, 1977; Pettit et al., 1984; Vaccarino et al., 1986). These studies demonstrate that some drugs of abuse may be reinforcing and may produce significant behavioral effects through DA-independent pathways. AAS may fall into this category.
AAS may act through pathways utilized by other non-stimulant drugs of abuse
While AAS do not act as stimulants, AAS share some properties with other drugs of abuse, particularly opiates. AAS induce autonomic depression quite similar to that induced by opiates (Peters and Wood, 2005). Interestingly, autonomic depressant effects of T, including the development of tolerance to locomotor, respiration, and body temperature suppression, can be blocked by naloxone-pretreatment. Furthermore, AAS self-administration is blocked by naltrexone-pretreatment. With chronic treatment, AAS can also alter endogenous opioid levels (Celerier et al., 2003; Johansson et al., 2000a; Johansson et al., 1997; Menard et al., 1995). It is tempting to speculate on how endogenous opioids may mediate the reinforcing properties of AAS. Nyberg and colleagues (Johansson et al., 2000a) hypothesized that AAS cause an imbalance in activation of μ-opioid receptors (MORs) in the VTA and κ-opioid receptors (KORs) in Acb, two prominent pathways through which endogenous opioids regulate mesolimbic DA system (Spanagel, 1995). While brains stem MORs likely mediate autonomic depressant effects of AAS, Acb KORs may play a central role in Acb DA and AAS self-administration. κ-agonists, like AAS, are mild reinforcers that reduce Acb DA (Marinelli et al., 1998) and known to reduce drug self-administration (Glick et al., 1995; Schenk et al., 1999). Thus, it is plausible that κ-opioids mediate Acb DA and AAS self-administration.
In addition to opiates, AAS also resemble GABAergic drugs of abuse. Both AAS and benzodiazepines do not increase Acb DA levels (Finlay et al., 1992; Invernizzi et al., 1991; Zetterstrom and Fillenz, 1990), despite their abuse potential. In addition, AAS have been shown to be anxiolytic (Agren et al., 1999; Aikey et al., 2002; Berbos et al., 2002; Frye and Seliga, 2001). Some of these acute behavioral effects may be mediated by T and its metabolites binding to the steroid binding site of the GABAa receptor (Masonis and McCarthy, 1995, 1996). In addition to their acute benzodiazepine-like effects on behavior, AAS have been shown to alter GABAa receptor (Clark and Henderson, 2003; Penatti et al., 2005), and glutamic acid decarboxylase 65 (Grimes et al., 2003) expression. It appears that AAS can modulate opioidergic and GABAergic systems, both acutely and chronically.
The possibility that AAS modulate pathways that mediate the effects of other drugs of abuse raises a concern that AAS abuse may alter vulnerability for the abuse of other drugs. In fact, there are reports suggesting that AAS abusers are more likely to use tobacco, alcohol, and illicit drugs than non-users (see Yesalis et al., 1993 for example). The evidence from animal studies suggest that AAS alter sensitivity to the rewarding effects of amphetamine (Clark et al., 1996) and increase EtOH intake (Johansson et al., 2000b). In addition to their recreational uses, it is not difficult to imagine use of AAS with other potentially addictive drugs for non-recreational purposes. For example, amphetamines and AAS may be taken by the same athletes in order to enhance their athletic performance. Also, AAS and opiates may be taken together following injuries, the former to hasten recovery (Ferry et al., 1999) and the latter for analgesia. This combination of opiates and AAS is particularly worrisome, since progestins, T, and related substances also have analgesic/anesthetic properties (Belelli et al., 2006; Frye et al., 2007; Frye et al., 1996; Weir et al., 2004). In fact, AAS may exacerbate morphine withdrawal symptoms (Celerier et al., 2003). Therefore, the underlying neural mechanism of AAS abuse and their interaction with other drugs of abuse warrant further investigation.
Acknowledgments
This research was supported by NIH grant DA-12843 to RIW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agren G, Thiblin I, Tirassa P, Lundeberg T, Stenfors C. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol Behav. 1999;66:503–9. doi: 10.1016/s0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–60. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol Biochem Behav. 2000;65:327–32. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Intracerebroventricular self-administration of commonly abused anabolic-androgenic steroids in male hamsters (Mesocricetus auratus): nandrolone, drostanolone, oxymetholone, and stanozolol. Behav Neurosci. 2005;119:752–8. doi: 10.1037/0735-7044.119.3.752. [DOI] [PubMed] [Google Scholar]
- Barreto-Estrada JL, Barreto J, Fortis-Santiago Y, Rivera-Ramos I, Fortis-Santiago A, Jorge JC. Modulation of affect after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Behav Neurosci. 2004;118:1071–9. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–9. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Berbos ZJ, Chu L, Wood RI. Acute behavioral effects of anabolic steroids: anxiety, stereotypy and locomotor activity. Horm Behav. 2002;41:457. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research - Brain Research Reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men.[see comment] N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Birgner C, Kindlundh-Hogberg AM, Nyberg F, Bergstrom L. Altered extracellular levels of DOPAC and HVA in the rat nucleus accumbens shell in response to sub-chronic nandrolone administration and a subsequent amphetamine challenge. Neurosci Lett. 2007;412:168–72. doi: 10.1016/j.neulet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatr Rep. 2002;4:377–87. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Bruning PF, Jonker KM, Boerema-Baan AW. Adsorption of steroid hormones by plastic tubing. J Steroid Biochem. 1981;14:553–5. doi: 10.1016/0022-4731(81)90029-7. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–22. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Yazdi MT, Castane A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol. 2003;465:69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic- androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–36. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Clark AS, Lindenfeld RC, Gibbons CH. Anabolic-androgenic steroids and brain reward. Pharmacol Biochem Behav. 1996;53:741–5. doi: 10.1016/0091-3057(95)02082-9. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Malott DH, Dickinson SD, Risinger FO. Haloperidol does not alter expression of ethanol-induced conditioned place preference. Behav Brain Res. 1992;50:1–5. doi: 10.1016/s0166-4328(05)80282-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–2. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeo AN, Wood RI. ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology. 2006a;31:237–49. doi: 10.1016/j.psyneuen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- DiMeo AN, Wood RI. Self-administration of estrogen and dihydrotestosterone in male hamsters. Horm Behav. 2006b;49:519–26. doi: 10.1016/j.yhbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision. American Psychiatric Association; Washington, DC: American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ferry A, Noirez P, Page CL, Salah IB, Daegelen D, Rieu M. Effects of anabolic/androgenic steroids on regenerating skeletal muscles in the rat. Acta Physiol Scand. 1999;166:105–10. doi: 10.1046/j.1365-201x.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21:189–94. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Damsma G, Fibiger HC. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology (Berl) 1992;106:202–8. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- Frye CA, Babson A, Walf AA. Self-administration of 3alph-androstanediol increases locomotion and analgesia and decreases aggressive behavior of male hamster. Pharmacol Biochem Behav. 2007;86:415–21. doi: 10.1016/j.pbb.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Frye CA, Reed TA. Androgenic neurosteroids: anti-seizure effects in an animal model of epilepsy. Psychoneuroendocrinology. 1998;23:385–99. doi: 10.1016/s0306-4530(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cognitive, Affective & Behavioral Neuroscience. 2001;1:371–81. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Rao PN, Erskine MS. Analgesic effects of the neurosteroid 3 alpha-androstanediol. Brain Res. 1996;709:1–9. doi: 10.1016/0006-8993(95)01118-8. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–52. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–80. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–8. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Pozzi L, Samanin R. Release of dopamine is reduced by diazepam more in the nucleus accumbens than in the caudate nucleus of conscious rats. Neuropharmacology. 1991;30:575–8. doi: 10.1016/0028-3908(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Johansson P, Hallberg M, Kindlundh A, Nyberg F. The effect on opioid peptides in the rat brain, after chronic treatment with the anabolic androgenic steroid, nandrolone decanoate. Brain Res Bull. 2000a;51:413–8. doi: 10.1016/s0361-9230(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Johansson P, Lindqvist A, Nyberg F, Fahlke C. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol Biochem Behav. 2000b;67:271–9. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K, Nyberg F. Anabolic androgenic steroids increase beta-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci Res. 1997;27:185–9. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. The ventral striatum of the Syrian hamster. Ann N Y Acad Sci. 1999;877:661–6. doi: 10.1111/j.1749-6632.1999.tb09296.x. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–92. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ., Jr Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther. 1983;227:229–37. [PubMed] [Google Scholar]
- Kindlundh AM, Rahman S, Lindblom J, Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci Lett. 2004;356:131–4. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–9. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–60. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Anabolic Steroid Abuse. NIDA Research Report Series. 2000:1–8. [Google Scholar]
- Marinelli M, Barrot M, Simon H, Oberlander C, Dekeyne A, Le Moal M, Piazza PV. Pharmacological stimuli decreasing nucleus accumbens dopamine can act as positive reinforcers but have a low addictive potential. Eur J Neurosci. 1998;10:3269–75. doi: 10.1046/j.1460-9568.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchis S, Salvador A, Moya-Albiol L, Gonzalez-Bono E, Simon VM. Effects of chronic treatment with testosterone propionate on aggression and hormonal levels in intact male mice. Psychoneuroendocrinology. 1998;23:275–93. doi: 10.1016/s0306-4530(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Masonis AE, McCarthy MP. Direct effects of the anabolic/androgenic steroids, stanozolol and 17 alpha-methyltestosterone, on benzodiazepine binding to the. gamma-aminobutyric acid(a) receptor. Neurosci Lett. 1995;189:35–8. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- Masonis AE, McCarthy MP. Effects of the androgenic/anabolic steroid stanozolol on GABAA receptor function: GABA-stimulated 36Cl- influx and [35S] TBPS binding. J Pharmacol Exp Ther. 1996;279:186–93. [PubMed] [Google Scholar]
- Menard CS, Hebert TJ, Dohanich GP, Harlan RE. Androgenic-anabolic steroids modify beta-endorphin immunoreactivity in the rat brain. Brain Res. 1995;669:255–62. doi: 10.1016/0006-8993(94)01266-k. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of The Golden Hamster Brain. Academic Press, Academic Press; 2001. [Google Scholar]
- Naruse T, Asami T. Intravenous self-administration of diazepam in rats. Eur J Pharmacol. 1987;135:365–73. doi: 10.1016/0014-2999(87)90686-8. [DOI] [PubMed] [Google Scholar]
- NHANES. [accessed 7/18/07];2007 www.cdc.gov/od/oc/media/pressrel/r041027.htm.
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci. 1997;111:219–24. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Packard MG, Schroeder JP, Alexander GM. Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of alpha-flupenthixol. Horm Behav. 1998;34:39–47. doi: 10.1006/hbeh.1998.1461. [DOI] [PubMed] [Google Scholar]
- Penatti CA, Porter DM, Jones BL, Henderson LP. Sex-specific effects of chronic anabolic androgenic steroid treatment on GABA(A) receptor expression and function in adolescent mice. Neuroscience. 2005;135:533–43. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Pert A, Sivit C. Neuroanatomical focus for morphine and enkephalin-induced hypermotility. Nature. 1977;265:645–7. doi: 10.1038/265645a0. [DOI] [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–81. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–73. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–82. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Dickinson SD, Cunningham CL. Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology (Berl) 1992;107:453–6. doi: 10.1007/BF02245175. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Rojas-Ortiz YA, Rundle-Gonzalez V, Rivera-Ramos I, Jorge JC. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Horm Behav. 2006;49:123–8. doi: 10.1016/j.yhbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Sato SM, Johansen J, Jordan CL, Wood RI. Androgen self-administration in Tfm rats. 10th Annual Meeting of Society for Behavioral Neuroendocrinology; Pittsburgh, PA. 2006. [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–46. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. European Journal of Endocrinology. 2003;148:281–92. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res. 1995;70:37–49. doi: 10.1016/0166-4328(94)00176-g. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC. A role for the mesolimbic dopamine system in the reinforcing properties of diazepam. Psychopharmacology (Berl) 1988;94:133–7. doi: 10.1007/BF00735894. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Kazandjian A, Varonos D. Diazepam-induced place preference conditioning: appetitive and antiaversive properties. Psychopharmacology (Berl) 1985;87:225–32. doi: 10.1007/BF00431813. [DOI] [PubMed] [Google Scholar]
- Szostak C, Finlay JM, Fibiger HC. Intravenous self-administration of the short-acting benzodiazepine midazolam in the rat. Neuropharmacology. 1987;26:1673–6. doi: 10.1016/0028-3908(87)90116-x. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br J Pharmacol. 1999;126:1301–6. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricker R, Casaburi R, Storer TW, Clevenger B, Berman N, Shirazi A, Bhasin S. The effects of supraphysiological doses of testosterone on angry behavior in healthy eugonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3754–8. doi: 10.1210/jcem.81.10.8855834. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Amalric M, Swerdlow NR, Koob GF. Blockade of amphetamine but not opiate-induced locomotion following antagonism of dopamine function in the rat. Pharmacol Biochem Behav. 1986;24:61–5. doi: 10.1016/0091-3057(86)90045-6. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–11. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41:247–58. doi: 10.1006/hbeh.2002.1769. [DOI] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–97. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- WorldAnti-DopingAgency. Adverse analytical findings reported by accredited laboratories. World Anti-Doping Agency; Montreal, Canada: 2006. [Google Scholar]
- Yesalis CE, Kennedy NJ, Kopstein AN, Bahrke MS. Anabolic-androgenic steroid use in the United States. JAMA. 1993;270:1217–21. [PubMed] [Google Scholar]
- Zetterstrom T, Fillenz M. Local administration of flurazepam has different effects on dopamine release in striatum and nucleus accumbens: a microdialysis study. Neuropharmacology. 1990;29:129–34. doi: 10.1016/0028-3908(90)90052-s. [DOI] [PubMed] [Google Scholar]