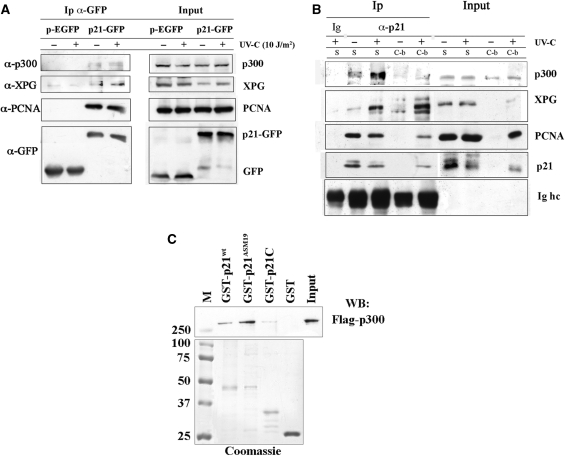
Figure 2.
p21 interacts and physically associates with p300 in vivo and in vitro. (A) Immunoprecipitation (Ip) with anti-GFP antibody on extracts from HeLa cells expressing pEGFP, or p21-GFP, before and 30 min after UV-C irradiation, as described in ‘Materials and Methods’ section. Input load: 1/30 of cell extracts. (B) Immunoprecipitation (Ip) with anti-p21 antibodies of soluble (S) and chromatin-bound (C-b) fractions from control and 30 min after UV-irradiation (10 J/m2) of LF1 fibroblasts. Immunoprecipitation with irrelevant antibody (Ig) is shown together with bands of immunoglobulin heavy chains (Ig hc) of anti-p21 antibodies. Input load: 1/30 and 1/15 of soluble or chromatin-bound fraction, respectively. (C) Direct association between recombinant p300 and p21. Equimolar concentrations (1 μM) of full-length GST-p21 (GST-p21wt), a p21 mutant that does not bind PCNA (GST-p21ASM19), a p21 C-terminal peptide (GST-p21C) or GST alone were pre-incubated with recombinant Flag-p300 protein (250 ng), and then pulled-down with GSH-agarose beads, as described in ‘Materials and Methods’ section. The lower portion of the gel shown was stained with Coomassie, while the upper part was analysed by Western blot (WB) with anti-Flag antibody. M: molecular weight markers (kDa).