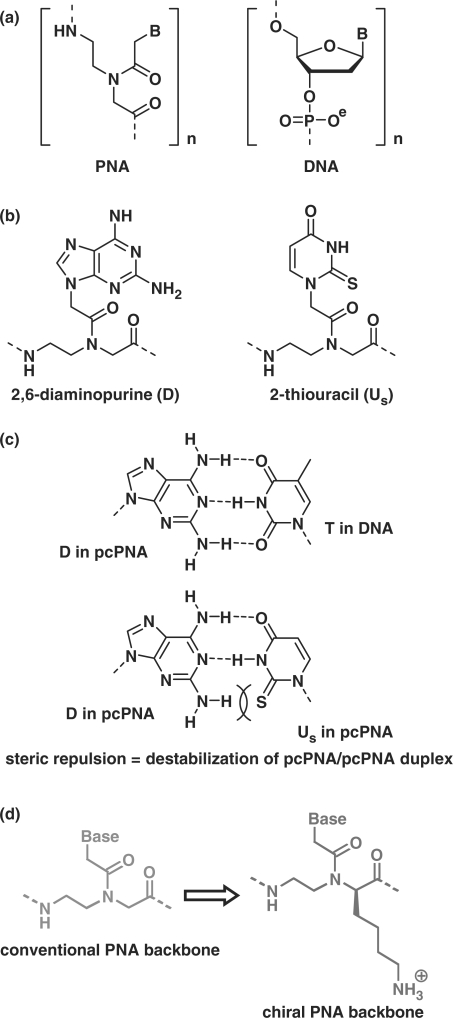
Figure 1.
(a) Chemical structures of PNA and DNA. (b) Pseudo-complementary bases 2,6-diaminopurine (D) and 2-thiouracil (Us) used in pcPNA. The D–Us pair is destabilized by steric repulsion between the 2-amino group of D and the 2-S atom of Us, although D–T and A–Us pairs are sufficiently stable, as shown in (c) (32). (d) Chiral PNA units from N-(2-aminoethyl)-d-lysine, used for the promotion of double-duplex invasion in place of conventional PNA units.