Abstract
The limbic system-associated membrane protein (LAMP) is a GPI-anchored cell adhesion molecule expressed heavily in limbic and limbic-associated regions of the developing and adult brain. Experimental studies show that LAMP promotes the growth of limbic neurons and guides the projections of limbic fibers. In order to examine the functional consequences of disrupting limbic circuit assembly, we generated a mouse line in which the Lsamp gene encoding LAMP was deleted. Basic neuroanatomical organization and sensory and motor development are normal in Lsamp−/− mice. The most profound change in behavior in both male and female Lsamp−/− mice is a heightened reactivity to novelty exhibited in several behavioral tests. Lsamp−/− mice display hyperactivity in a novel arena and both sexes habituate to the same activity levels as their wildtype littermates, but at different rates. In the elevated plus maze, Lsamp−/− mice exhibit increased total arm entries, with a bias towards the open arms; they spend more time in the open arms and have a substantial increase in the amount of risk assessment in unprotected areas of the maze. In the y-maze, Lsamp−/− mice exhibit characteristic hyperactivity and a decreased level of spontaneous alternation during the period when their novelty-induced hyperactivity is at its peak. We hypothesize that Lsamp−/− mice may not simply exhibit a decrease in anxiety, but may have a heightened, and possibly maladaptive, response to novel environmental stressors. Genetic deletion of Lsamp may thus cause circumscribed changes in the fine connectivity of specific circuits that underlie these behaviors.
Keywords: LAMP, limbic system, hyperactivity, anxiety, cell adhesion molecule, knock-out mouse
1. Introduction
The limbic system is comprised of interconnected brain structures responsible for emotional regulation, cognitive function and autonomic responses. Although first described more than 120 years ago [4], hypotheses regarding the functional organization and specific contributions of the basic circuitry to complex behaviors are under continuous refinement. There is even debate regarding the limbic system as a unifying concept [11,17]. Yet, specific frontal and temporal cortical areas, forebrain regions (septum, amygdala, hypothalamus) and brainstem nuclei (locus coeruleus, raphe, vagal nuclei) are implicated in the behavioral and physiological disruptions that cause neuropsychiatric diseases such as anxiety, depression and psychosis. However, the underlying, complex changes at the circuit level remain ill-defined [34]. A neurodevelopmental etiology has been hypothesized for many psychiatric disorders [1,15,30,51], suggesting that the functional impact may occur through the disruption of the assembly of limbic circuitry. One approach to examine the development, maintenance and disruption of limbic-related behaviors is to manipulate the expression of molecules that mediate the development and function of the underlying neural circuitry.
The limbic system-associated membrane protein (LAMP), a cell adhesion molecule (CAM) of the IgLON family expressed in cortical and subcortical limbic-associated regions of the developing and adult brain [5,6,20,29,39,44,54] is one such molecule. The protein exhibits 99% homology between rodent and human [38] and there is a close correlation between Lsamp mRNA and protein distribution patterns in rat [29,39,44,54], monkey [5,6], and human [42,43]. Experimental manipulations of LAMP in vitro result in altered axon targeting and neurite growth [13,24,31,41,56]. In the analysis of different rat substrains, Nelovkov and colleagues correlated lower expression of Lsamp mRNA in the amygdala and hippocampus with decreased anxiety and increased exploration [35,36]. Moreover, there is genetic association of a polymorphism in the Lsamp gene with panic disorder in humans [26,32]. Both studies suggested that alterations in LAMP may have functional consequences on complex behaviors.
We have developed mice in which the Lsamp gene is deleted constitutively. Here, we have evaluated gross neuroanatomical organization and characterized the behavioral phenotype of Lsamp−/− mice by providing an assessment of their response to novel, stressful environments as measured by activity and exploratory behavior. The results of this study support the importance of LAMP in limbic function and provide the basis for further anatomical, physiological and biochemical phenotyping of Lsamp−/− mice.
2. Materials and Methods
2.1 Lsamp targeting
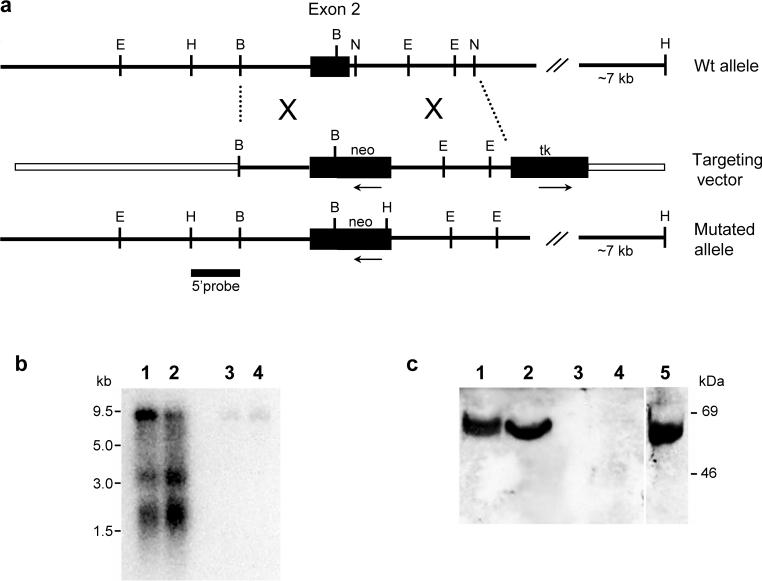
To disrupt the Lsamp gene, we generated a targeting vector that replaced 69 nt of exon 2 including the 3’ splice site and 31 nt of intron 2 with a neo cassette inserted in the opposite transcription/translation frame relative to the Lsamp gene (Fig.1a). To generate the targeting vector, a mouse 129/ReJ genomic library constructed in the λ FIX II vector (provided by Dr John Pintar, UMDNJ-RWJMS, Piscataway, NJ) was screened using probes derived from the rat Lsamp cDNA [41]. Among the Lsamp genomic clones isolated and characterized, the mLsamp-λ-11a clone was selected for containing a 13.2 kb insert, including the partial nucleotide sequence of the first intron, exon 2 and ∼4.0 kb of intron 2. The linearized vector was electroporated into R1 ES cells ((129X1/SvJ × 129S1/Sv)F1-Kitl+). Targeted ES cells were injected into C57BL/6J blastocysts to generate chimeric mice. One heterozygous Lsamp founder was obtained and back-crossed into the C57BL/6J strain for more than 10 generations for all experiments reported here. Initial genotyping was done by Southern Blot analysis. All subsequent mice were genotyped by polymerase chain reaction (PCR) amplification using primers to identify the presence of the wild type allele (5`- GTC CTG ATT GGT CTT GTT GAG TCC -3` and 5`-TCT TAT CCC ACT TCC CCC TTA CC -3`) and the targeted allele (5`-CTC CTG CCG AGA AAG TAT CCA TC-3` and 5`-CTC TGG AAT ACA GCC TCC GAA TC-3`). PCR reactions were performed using the AmpliTaq gold system (Applied Biosystems, Foster City, CA).
Figure 1. Targeted disruption of the Lsamp gene.
(a) Restriction map of the Lsamp+/+ genomic nucleotide sequence surrounding Lsamp exon 2 indicates the region of homology selected for construction of the targeting vector. The schematic representation of the mutated allele represents the homologous recombination event that disrupted the Lsamp locus. The location of the 5’ probe used for the screening of the targeted event is indicated. (b) Northern blot of Lsamp+/+ (lanes 1,2) and Lsamp−/− (lanes 3,4) mRNAs from hippocampus (lanes 1,3) and cerebellum (lanes 2,4). Three bands representing different sized Lsamp transcripts are evident in the Lsamp+/+ samples. Note the absence of message in the samples harvested from null mice. (c) Membrane extracts from cerebellum (lanes 1,3) and hippocampus (lanes 2,4) were analyzed by Western blotting. Samples from Lsamp+/+ mice (lanes 1,2) exhibit a single band of approximately 64−68kD, whereas samples harvested from Lsamp−/− mice (lanes 3,4) do not have this band. As a control, lane 5 depicts LAMP recombinant protein that is the same molecular mass as the native protein. Abbreviations for restriction enzymes: B, BamH I; E, EcoR I; H, Hind III; N, Nco I.
GeneBank accession number for the rat Lsamp nucleotide sequence is U31554. LSAMP/Lsamp are respectively the symbols for the human and rodent gene encoding LAMP, its mRNAs and cDNAs, approved by the human and mouse gene nomenclature committees. LAMP is the designation for the protein [54].”
Unless otherwise indicated, all standard molecular biology techniques were performed as described by Ausubel et. al. [2] and Sambrook et. al. [48]. All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted following the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80−23, revised 1996).
2.2 Northern Blot Analysis
Total cellular RNA was isolated from adult mouse hippocampus and cerebellum using the TRIzol reagent (Invitrogen, Carlshad, CA) following manufacturer's protocol. The poly(A)+ RNA fraction was purified using the Oligotex mRNA isolation system (Qiagen). Poly(A)+ RNA (1 μg) was separated on a 1.5% agarose-formaldehyde gel, transferred to a nylon membrane (Nytran SuperCharge, Schleicher and Schuel, Keene, NH), UV cross-linked, and hybridized overnight under stringent conditions with 32P-labeled cRNA probes. Antisense probes were transcribed in vitro using T7 RNA polymerase from rat Lsamp cDNA template [41] linearized with MscI (nt 464−1238). GeneBank accession number for the rat Lsamp nucleotide sequence is U31554.
2.3 Western Blot Analysis
Crude membrane preparations of hippocampus and cerebellum from adult mice were solubilized with 4% CHAPS [54,55], separated on 10% PAGE using standard methods [27] and blotted onto nitrocellulose membranes. LAMP immunoreactivity was detected using a chicken anti-LAMP polyclonal antibody, produced by Ames Laboratory (Tigand, OR) against recombinant protein that was purified to homogeneity in our laboratory. Specificity of the purified IgY fraction was characterized in our laboratory using Western Blot analysis. This polyclonal antibody specifically recognizes recombinant LAMP as well as a single, 64−68kDa band corresponding to native LAMP from crude membrane brain extracts.
2.4 Histological Analysis
Standard cresyl violet and Kluver-Barrera stains [25] were used for analysis of general gray matter cytoarchitecture and myelination. A monoclonal antibody (4A11) that recognizes neurofilament-H (NF-H) [40] was used to map the general organization of forebrain fiber tracts. The antibody was used at a 1:100 dilution, followed by a standard HRP/DAB reaction [40]. Acetylcholinesterase histochemistry [45] was used for the assessment of the organization of the septo-hippocampal cholinergic pathway. The fixation, sectioning of tissue and all standard histological procedures and stains, unless otherwise indicated, were performed as described by Hockfield et al. [18]. Complete serial sections from 3−5 animals of each genotype were examined at postnatal day (P) 6, P16 and adults.
2.5 Behavior
As noted above, all adult mice used for the behavior studies were backcrossed for more than 10 generations the C57BL/6J background. Mice were housed on ventilated racks in Plexiglas shoebox cages filled with CareFresh shredded paper bedding (Absorbtion Corp., Bellingham, WA). Mice were housed in groups of 2−5 per cage and given access to food (Lab Diet Rodent Chow 5001, PMI Nutrition International, Brentwood, MO) and water ad libitum. The colony was temperature (22.22±1°C) and light controlled (12 hour light/dark cycle, lights on at 6 a.m. CST). All animals for these studies were obtained by heterozygous breedings. Lsamp−/− mice and their wildtype (Lsamp+/+) littermates were used for all behavioral tests. Behavioral testing was performed in the Vanderbilt Kennedy Center and Center for Molecular Neuroscience Murine Neurobehavioral Core. Mice were tested between 3−6 months of age and were behaviorally naïve at the time of testing except for those in the 30 minute activity chamber experiment (see section 2.5.3.2). In all adult testing, males and females were tested in separate groups. All testing took place between 12−6 p.m. Housing and testing procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. For all behavioral testing, sample sizes for each genotype and sex ranged from 7−16 animals/group. Specific group sizes are noted for each test in the figure legends. Strategies for analytical and statistical procedures were developed with consultants in the Vanderbilt Kennedy Center Statistics and Methodology Core. In all cases where coding was done by hand, coders (blind to genotype) achieved inter-rater reliability of greater than 95%. In all tests where there was a main effect of sex, the sexes were then split, a new omnibus was performed and subsequent analysis was performed separately.
2.5.1 Postnatal development
The first litters born from Lsamp heterozygous breeding pairs were monitored daily in their homecage during the first postnatal week for nesting, feeding and postnatal lethality. Sensorimotor responses and body weight were used to assess the postnatal development of the Lsamp mice. Mice were tested and scored at P3-P5, P7, P14 and P21, as described by Fox [14]: righting reflex, postural flexion and extension, limb grasping, negative geotaxis, bar holding, cliff drop-aversion, tail suspension and visual placing.
2.5.2 Acoustic startle and prepulse inhibition
2.5.2.1 Apparatus
The acoustic startle reflex and prepulse inhibition (PPI) of the acoustic startle reflex were evaluated using four identical, ventilated, sound-attenuated acoustic chambers (51×55×31 cm; Med Associates, St. Albans, VT, USA), each equipped with two speakers, a mouse holder and a transducer system through which startle responses were recorded. Chambers were connected to an amplifier and to a computer equipped with the Startle Reflex software (MED Associates).
2.5.2.2 Testing Procedures
Group housed mice were handled for the three days preceding testing and were acclimated for one hour in an adjacent room on testing day. PPI was performed following the behavioral core protocol as previously described [21]. Each mouse was placed in a holder that was then mounted on the response platform. Test sessions were preceded by a 5 minute acclimation period in the startle chambers during which a 65-dB background noise was continuously present, followed by 54 trials in 9 blocks of 6 trials each. Each six-trial block contained one startle trial (40-ms, 120-dB burst of white noise), one null trial (no stimulus) and four prepulse trials (20-ms bursts of 70-, 76-, 82-, and 88 dB white noise; followed 100 ms later by the startle stimulus). The trials in which no stimulus was presented were used to measure baseline movement in the cylinders. The six different trial types were pseudo-randomly assigned. The inter-trial interval ranged from 10−20 s with an average of 15s. Mice were exposed to the EPM for 5 minutes ten days prior to this test.
2.5.2.3 Analysis and Statistics
PPI was calculated as the percent reduction in maximal startle on prepulse versus startle only trials. PPI data were analyzed using repeated measures ANOVA.
2.5.3 Activity Chamber
2.5.3.1 Apparatus
The activity chambers (MED Associates, Georgia, VT) were square arenas (27cm × 27cm × 20cm) with clear Plexiglas walls and white floors. An infrared beam break system positioned 1 cm above the floor on both the x and y axis was used to monitor mouse horizontal movements.
2.5.3.2 Testing Procedures
Group-housed mice were handled once daily for the three days preceding testing. Mice were transported into the testing room one at a time from an adjacent room, placed in the middle of the novel activity chamber and allowed to explore the chamber for 30 minutes. The chambers were illuminated at 550−650 lux and a white noise generator was placed in the room. Activity chambers were cleaned with water and 70% ethanol between each animal. Male and female mice were run on the y-maze for 8 minutes one week prior to this experiment. Exploration and alternation in the y-maze is dependent on novelty; therefore, y-maze was run prior to measuring activity. However, a second cohort of behaviorally naïve, unhandled male mice was used to monitor activity over a one-hour time period.
2.5.3.3 Analysis and Statistics
The beam break data collected using the Med Associates software was used to measure the total distance (cm) traveled per ten-minute block. For the 30-minute trial a repeated measures ANOVA was used with genotype and sex as between subject factors and distance traveled as within subjects factor. For the 1-hour test (males only) data were analyzed using repeated measures ANOVA with genotype as the between subjects factor. If the omnibus test detected a significant effect for genotype, or genotype*behavior, a post-hoc t-test was performed to determine at which time points the differences occurred.
2.5.4 Elevated Plus Maze
2.5.4.1 Apparatus
The elevated plus maze (EPM) was a plus shaped apparatus consisting of two open arms (platforms with no sides) and two closed arms (platforms with tall walls) connected by a small center square. Both the open and closed arms of the maze were 30 cm long × 5 cm wide with white Plexiglas floors. The closed arms had 15 cm high walls made of black Plexiglas and the open arms were equipped with a 0.25cm high Plexiglas edge on the sides and ends to decrease the chance of mice falling off the maze. The center box was 5 × 5 cm. The maze was built on 40 cm high legs and placed on the floor for testing. Four white screens were placed around the maze in order to reduce spatial cues from the room.
2.5.4.2 Testing Procedures
In all EPM experiments, animals were brought into the testing room one at a time from a neighboring room, placed in the center of the maze and allowed to freely explore for 5 minutes. Mice were naïve and not handled prior to exposure to the EPM. A white noise generator was present in the room for all experiments and a camera was placed directly above the maze to record the behavior of each animal. The maze was illuminated at approximately 250 lux. The maze was cleaned with water and 70% ethanol between each animal. Additional cohorts of animals were run under dimmer lighting conditions (∼ 60lux) and after being handled, but there was no statistical effect of these environmental manipulations on any of the standard EPM measures.
2.5.4.3 Analysis and Statistics
The number of entries into the open and closed arms, and duration of time spent in open arms, closed arms and center of the maze were measured for each animal. Entries and exits from maze arms were defined as all four paws crossing into or out of the arm. Additionally, as an indication of risk assessment, we measured both unprotected and protected head-dipping. Unprotected head-dips were defined as the head, neck and shoulders of the mouse crossing off the edge of an open arm while all four paws were in an open arm. Protected head-dips were defined as the head, neck and shoulders of the mouse crossing off the edge of an open arm while at least one paw was in either the center or closed arms of the maze. Entries and durations measurements were automated using the MazeScan suite of TopScan video analysis software (CleverSys Inc., Reston VA). TopScan measurements were validated by comparison to hand scoring by a trained, observer blind to genotype. The hand coding was performed from video using ProcoderDV (Vanderbilt University, Nashville TN) and correlations between TopScan and hand coding were greater than 0.90 for all measurements. A trained observer blind to genotype manually coded head-dips. Each of the three specific behavior categories (entries, duration and head-dips) was analyzed separately. An omnibus repeated measure ANOVA was performed for each with genotype and sex as between subject factors and area of the maze (i.e. open/closed arm, protected/unprotected area) as the within subject factors. To control for analyzing data from multiple EPM measures, a Bonferroni-corrected alpha (0.0167) was used. Significant genotype or genotype*behavior effects were followed by post-hoc t-tests.
2.5.5 Y-maze
2.5.5.1 Apparatus
The y-maze was a y-shaped apparatus in which the three arms were of equal length. The three enclosed arms were made of clear circular Plexiglas and the bottom of the Plexiglas tube was removed so that the maze sat flat on a grey rubber surface. Each arm was 30.5cm long × 4.8 cm wide × 4.3 cm tall. The end of one arm was removable for placement of mice in the maze. The arms of the maze joined in the center with each arm at a 120° angle from the next. Spatial cues were available to the mice during testing (e.g. walls, door to room, shelving in room).
2.5.5.2 Testing Procedures
Male and female mice were naive prior to the y-maze test. All mice were handled for three days prior to testing. Mice were transported into the room one at a time from a neighboring room and placed in the end of one arm of the y-maze. They were allowed to freely explore the chamber for 8 minutes. A camera was placed directly above the maze to record the animal's behavior. The room was illuminated at approximately 200 lux. The maze was cleaned with water and 70% ethanol between each animal.
2.5.5.3 Analysis and Statistics
A trained observer blind to genotype scored the number and sequence of arm entries in the y-maze. An arm entry was defined as all four paws crossing into an arm. The number of spontaneous alternations, same arm returns and alternate arm returns were then determined. Spontaneous alternations were defined as entries into each of the three arms in a sequential manner. Same arm returns (SAR) were defined as re-entering the same arm that was just visited after all four paws left the arm and before any other arm was entered. Alternate arm returns (AAR) were defined as entry into two of three arms in three sequential entries where the same arm was entered at the beginning and end of the triplet (e.g. arm A,B,A). Percent spontaneous alternation for each animal was calculated as the ratio between the number of actual spontaneous alternations (#SA) and the total number of possible spontaneous alternations (total entries – 2) multiplied by 100: (#SA/(total entries-2))*100. Alternate arm returns and same arm returns were calculated as a ratio of returns to total number of entries multiplied by 100 (e.g. (AAR/total entries)*100). A MANOVA was calculated using four measures (total entries, %SA, %AAR and %SAR) including sex and genotype as factors.
3. Results
3.1 Initial characterization of Lsamp−/− mice
The targeting event resulted in disruption of the Lsamp locus (Fig. 1a). As a consequence of this genetic manipulation, the mutant mice lack both Lsamp transcripts and LAMP protein as determined by Northern and Western Blot analysis, respectively (Fig. 1b and c). Analysis of over 250 litters, congenic into C57BL/6J strain, revealed an expected Mendelian ratio of +/+, +/− and −/− genotypes, reflecting normal viability in utero and postnatally. Lsamp −/− mice were normal in appearance, size, growth and development. There were no differences in monitored weight gain and brain weight was identical for Lsamp −/− and Lsamp +/+ male littermates (Table 1). The gross anatomy of the brain was normal. Thus, white matter staining, general gray matter cytoarchitecture and the appearance of forebrain cortical and subcortical structures did not differ across genotypes. For this initial screening, several immuno- and histochemistry stains were used. Normal cytoarchitecture and fiber tracts are illustrated in Figure 2. Cortical lamination patterns and amygdala and thalamic nuclei all appear intact and well-delineated. In addition, commissural pathways throughout the forebrain were intact. AChE histochemistry indicated a normal patterning of the septo-hippocampal cholinergic pathway, differing from experimental studies in vitro [24,55]. Normal AChE reactivity (Fig. 2c) is depicted in the hippocampus, amygdala, caudal striatum, limbic thalamic nuclei and lateral hypothalamus. Strong reactivity identifies cholinergic fibers in the internal capsula and the mammillothalamic tract in both genotypes. Thus, there currently is no indication from basic neuroanatomical examination of major alterations in brain organization and gross connectivity in the Lsamp−/− mouse.
Table 1.
Lsamp −/− mice are fertile and show normal growth.
| Measures | Lsamp−/− | Lsamp+/+ |
|---|---|---|
| Body weight, P6 | 3.5 +/− 0.6 (n=13) | 3.6 +/− 0.4 (n=10) |
| Body weight, P7 | 4.0 +/− 0.5 (n=36) | 4.1 +/− 0.7 (n=53) |
| Body weight, P14 | 7.4 +/− 1.0 (n=19) | 7.2 +/− 0.6 (n=20) |
| Body weight, P21 | 10.0 +/− 1.4 (n=6) | 10.4 +/− 1.2 (n=12) |
| Body weight, Adult males | 29.3 +/− 2.0 (n=19) | 28.4 +/− 2.0 (n=17) |
| Body weight, Adult females | 22.1 +/− 1.7 (n=50) | 22.5 +/− 1.9 (n=52) |
| Brain weight, Adult male | 0.48 +/− 0.01 (n=20) | 0.48 +/− 0.02 (n=20) |
| Fertility | Male and Female mice are fertile | |
| Nesting and feeding (P0-P7) | All mice in nest; milk plaque present | |
| Lethality | No neonatal lethality associated with genotype | |
Body and brain weight values in grams
Figure 2. Histological analysis of adult Lsamp−/− mice.
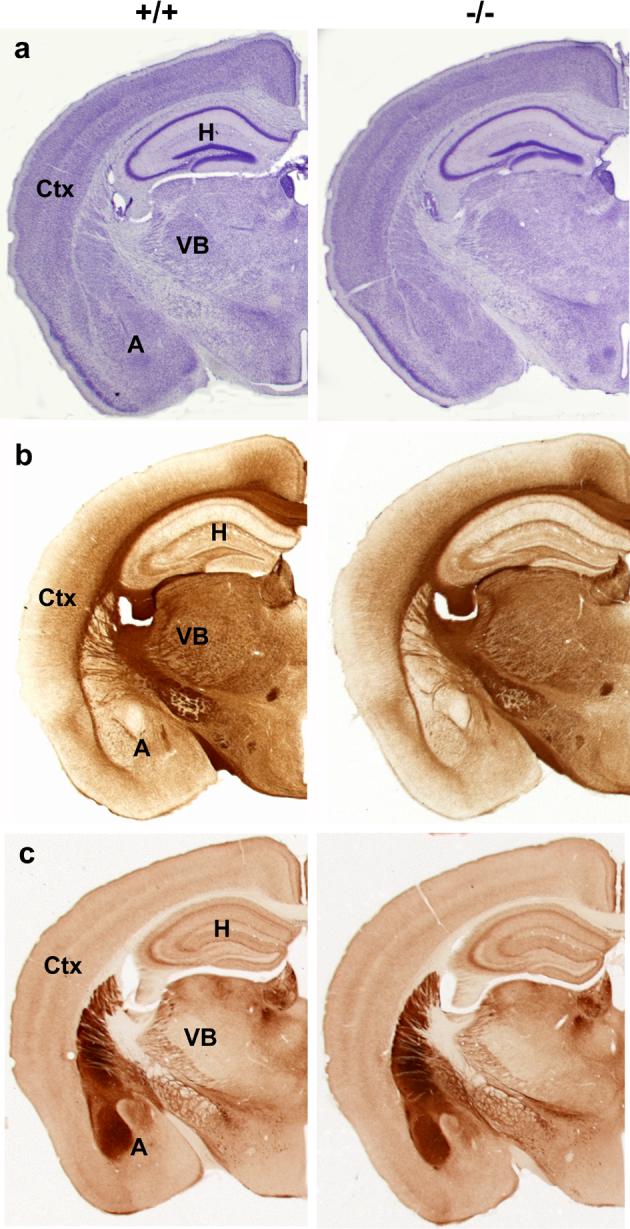
Coronal section images taken at the level of rostral hippocampus of Lsamp+/+ and Lsamp−/− littermates. Note the normal cytoarchitecture of the cerebral cortex (ctx), hippocampus (H), amygdala (A), thalamic ventral basal complex (VB) and hypothalamus viewed by cresyl violet staining (a). Major fiber tracts, viewed by immunostaining with a neurofilament-H antibody (b), also appear normal in Lsamp−/− mice compared to their Lsamp+/+ counterparts. Limbic structures and fiber tracts show normal distribution of AChE histochemistry in both genotypes (c). N=5/genotype for each histological staining. Scale bar =1mm.
3.2 Behavioral Analysis
Lsamp−/− mice were indistinguishable from littermates from the day of birth. The overall motor and sensory development of Lsamp−/− mice was evaluated from P3-P21 using a selected battery of tests [14]. In all sensorimotor tests used, Lsamp−/− mice acquired and performed mature responses at the same rate and ability as did their wildtype littermates (Table 2), demonstrating normal reflex maturation and normal gross sensory and motor abilities.
Table 2.
Sensory and motor development of Lsamp −/− mice.
| Measures of development | Mature Responses in Lsamp−/− mice | ||||
|---|---|---|---|---|---|
| P5 | P7 | P14 | P21 | Adult | |
| Righting | 85 | 100 | |||
| Postural flexion/extension | normal | normal | |||
| Forelimb grasping | 100 | 100 | |||
| Hindlimb grasping | 100 | ||||
| Inverted screen holding | 100 | 100 | |||
| Negative geotropism | 100 | 100 | |||
| Bar holding | 100 | 100 | |||
| Cliff drop aversion | 100 | 100 | |||
| Tail suspension | 100 | 100 | |||
| Visual Placing | 100 | ||||
| Eyelid opening | open | ||||
| Startle | normal | ||||
| Pre-pulse inhibition | normal | ||||
Acquisition of mature responses in Lsamp −/− are expressed as percentage of animals expressing a mature response (Score = 9). No differences found between genotypes.
3.2.1 Acoustic startle response and prepulse inhibition of acoustic startle
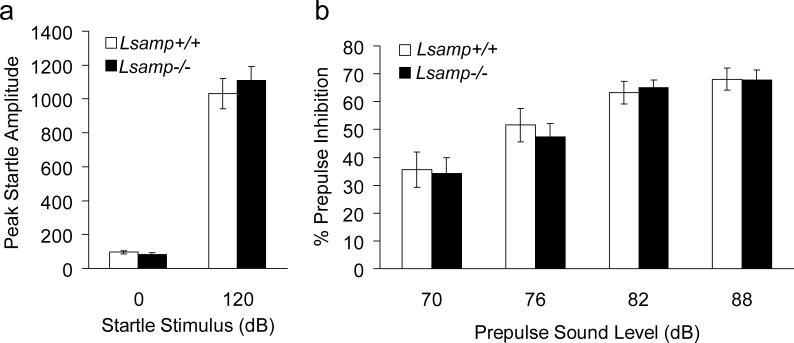
The acoustic startle response and sensory motor gating were evaluated in adult mice. There was no difference in the amplitude of the startle responses between Lsamp −/− and Lsamp +/+ mice, and no deficits in PPI (Fig. 3). In addition, no differences in baseline levels of movement were found between genotypes in the “no stimulus” trials (Fig. 3a). There was a significant decrease in startle with increasing prepulse stimuli in both genotypes (F(3,78)= 33.38, p < 0.0001), demonstrating the effectiveness of the PPI protocol (Fig. 3b). These data are consistent with intact circuitry involved in the acoustic startle reflex and in those circuits which modulate PPI in the Lsamp −/− mice.
Figure 3. Normal acoustic startle response and sensorimotor gating in Lsamp−/− mice.
Startle amplitude (a) and prepulse inhibiton of acoustic startle responses over varying prepulse intensities (b) are shown for Lsamp−/− mice (closed bars) and wildtype littermates (open bars) represented as mean±SE (n=14/group).
3.2.2 Activity Chamber
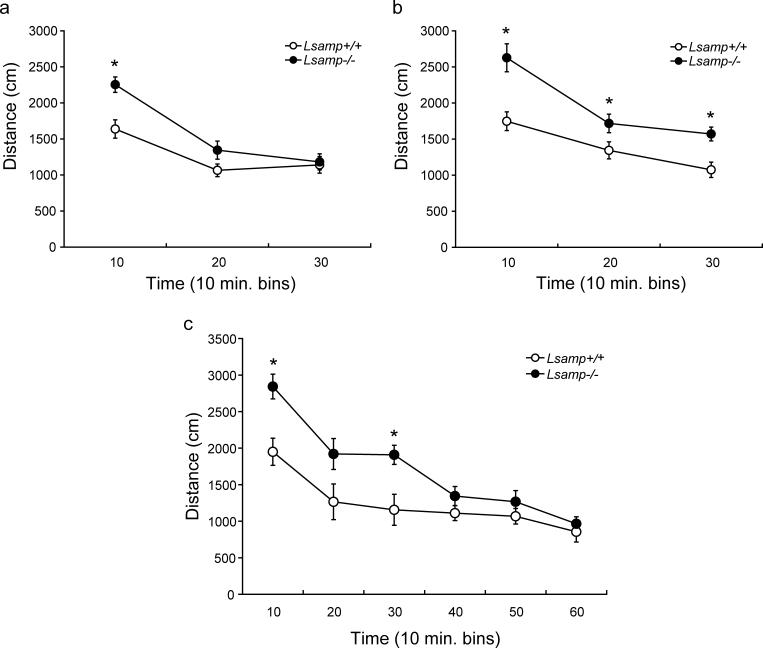
Lsamp−/− mice displayed hyperactivity during exposure to a novel open arena. Activity data during the 30-minute test for females and males are displayed in Figure 4. The initial repeated measures ANOVA demonstrated main effects of sex (F(1,54)=5.47, p = 0.023), genotype (F(1,54)=18.58, p < 0.0001), and distance traveled over time (F(2,54)=129.21, p < 0.0001). There also was a significant interaction of time × genotype (F(2,108)=11.172, p < 0.0001). Because there was an effect of sex, male and female data were split for the subsequent analyses. There was a main effect of genotype on distance traveled over time for both females (F(1,28)=4.84, p = 0.0002) and males (F(1,26)=14.73, p= 0.0007), and an interaction of genotype × distance traveled (F(2,56)=10.33, p= 0.0001 and F(2,52)=4.10, p = 0.022, respectively). Both female and male Lsamp−/− mice traveled a significantly greater distance than did their Lsamp +/+ littermates during the first ten minutes of the test (t(28)= 3.71, p = 0.0009 and t(26)= 3.86, p = 0.0007). During the second and third ten-minute blocks of the test, female Lsamp −/− mice habituated to normal activity levels and did not significantly differ from Lsamp +/+ during either time block (Fig. 4a). However, male Lsamp −/− mice continued to display hyperactivity throughout the remainder of the 30-minute test (Fig. 4b).
Figure 4. Open field activity of Lsamp−/− mice.
The distance traveled, measured in centimeters (cm), by Lsamp−/− mice and their Lsamp+/+ littermates is displayed in 10 minute bins (a&b). Both female (a) and male (b) Lsamp−/− mice exhibit hyperactivity when exposed to the novel arena. Female Lsamp−/− mice habituate to normal levels of activity within 20 minutes of being placed in the arena, but male Lsamp−/− mice remain hyperactive for the duration of the 30 minute test. (n=13−15/group). Examination of activity in the open field for a 60 minute period (c) revealed that the null mice return to normal levels of activity by the second half of the test period. The increase in distance traveled by male Lsamp−/− mice during the second 10 min bin (20) was not significant at p = 0.06 (n=7 WT, n=8 Lsamp−/−).*p<0.05
The highest levels of hyperactivity occured during the first 10 minutes in both female and male Lsamp −/− mice. Female Lsamp −/− habituated to their environment during the 30-minute testing period. We thus hypothesized that the male Lsamp −/− mice may also habituate to normal activity levels if provided with an extended testing period. Therefore, we repeated the experiment with a new group of males but extended the length of time in the chamber to one hour (Fig 4c). Again, there were main effects of genotype (F(1,13)=7.04, p = 0.0199), distance traveled over time (F(5,13)=42.39, p < 0.0001) and an interaction between the two (F(5,65)=4.37, p = 0.0017). In the new group of mice, the period of greatest hyperactivity also occurred during the first 10 minutes of exposure to the novel chamber (t(13)= 3.56, p = 0.0035) and the male Lsamp −/−mice still displayed hyperactivity 30 minutes into the test (Fig 4c). During the ten minute bins of the final 30 minutes of the test, however, Lsamp −/− mice did not travel a greater distance than did their Lsamp +/+ littermates, indicating that like the females, the male Lsamp −/− mice eventually habituated to the novel environment.
3.3 Elevated Plus Maze
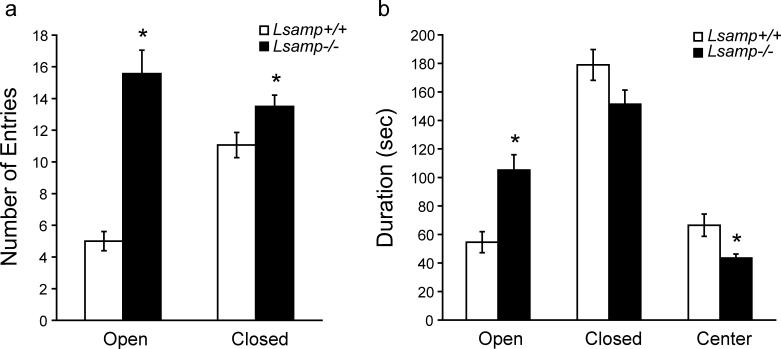
There was no main effect of sex on either of the traditional measures used for EPM (entries and durations); therefore the sexes were combined for the remainder of the analyses. The results of the EPM are presented in Figures 5 (entries, duration) and 6 (head-dips). There was a main effect of genotype (F(1,28)=39.44, p < 0.0001) on the number of entries and an interaction between category of entry and genotype (F(1,28)=22.37, p < 0.0001). Lsamp−/− mice demonstrated an increased number of entries into both the open (t( 30)=6.59, p < 0.0001) and closed (t(30)=2.27, p < 0.031) arms of the maze (Fig 5a). The Lsamp +/+ mice made more than double the number of closed entries as open entries (means±SE of 11±0.8 vs. 5±0.6), whereas Lsamp −/− mice made a similar number of entries into the closed and open arms (14±0.72 and 16±1.5). There was no main effect of genotype on durations; however, there was an interaction between genotype and category of duration (F(2,56)=67.20, p < 0.0001). This interaction occurred because Lsamp −/− mice spent more time in the open arms (t(30)= 3.88, p = 0.0005), with a corresponding decrease in center time (t(30)=2.78, p = 0.0093), but no significant difference in time spent in the closed arms of the maze (Fig. 5b).
Figure 5. Lsamp−/− mice behavior on the EPM.
Lsamp−/− mice make a significantly greater number of entries into both the open and closed arms of the EPM (a), indicating hyperactivity. Lsamp−/− mice also reside for a longer duration (sec) in the open arms of the maze, with a corresponding decrease in the amount of time spent in the center (b). Because there was no effect of sex on traditional EPM measures, male and female mice were combined in these analyses. (n=16/group)*p<0.01
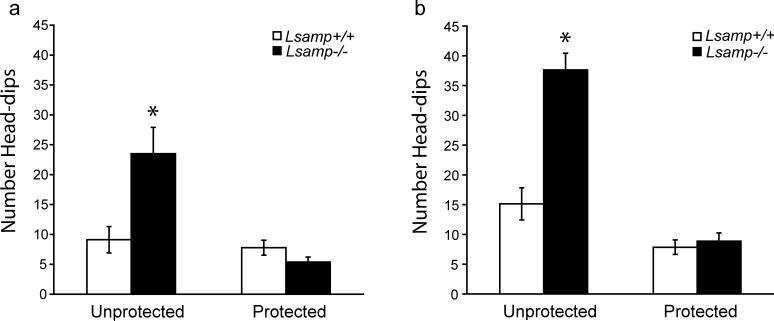
Figure 6. Exploratory behavior of Lsamp−/− mice on the EPM.
Measures of head-dips were quantified as a measure of risk assessment. Both female (a) and male (b) Lsamp−/− mice make more than double the number of unprotected head-dips, compared to their Lsamp+/+ littermates. There is no difference in the number of protected head-dips in null mice of either sex. (n=7−9/group)*p<0.01
Risk-assessment behavior on the EPM also was monitored by examining head-dips. There were main effects of sex (F(1,28)=11.31, p = 0.0022), genotype (F(1,28)=25.28, p < 0.0001) and area of the maze (F(1,28)=75.38, p < 0.0001) on the number of head-dips. The omnibus test also detected interactions between genotype and area of the maze (F(1,28)=35.82, p < 0.0001), and sex and area of the maze (F(1,28)=6.73, p < 0.0149). Since there was a main effect for sex, we split males and females for subsequent analyses on the contribution of genotype to altered head-dipping behavior. Omnibus testing of each sex separately still detected an effect of genotype and an interaction between genotype and area of the maze in which head-dips occurred. Both female and male Lsamp −/− mice demonstrated a large increase in the number of unprotected head-dips (t(15)=3.02, p = 0.0086 and t(13)=5.70, p < 0.0001), while maintaining a normal number of protected head-dips as compared to Lsamp +/+ littermates (Fig 6). Because the EPM was the only test in which animals were not handled before being exposed to the apparatus, we repeated the test with both handled male and female mice under slightly different environmental conditions (new room and reduced light levels). We combined data and analyzed across groups (taking into account sex, handling, and testing environment) and genotype. There was no effect of testing procedures on either entries or duration and the effect of genotype remains highly significant for both measures (F(1,28)=6.73, p < 0.0001 and F(1,28)=6.73, p < 0.0001). Thus, regardless of environmental manipulations, Lsamp −/− mice displayed hyperactivity, increases in open arm time, increases in open arm entries and increases in unprotected head-dips.
3.4 Y-maze
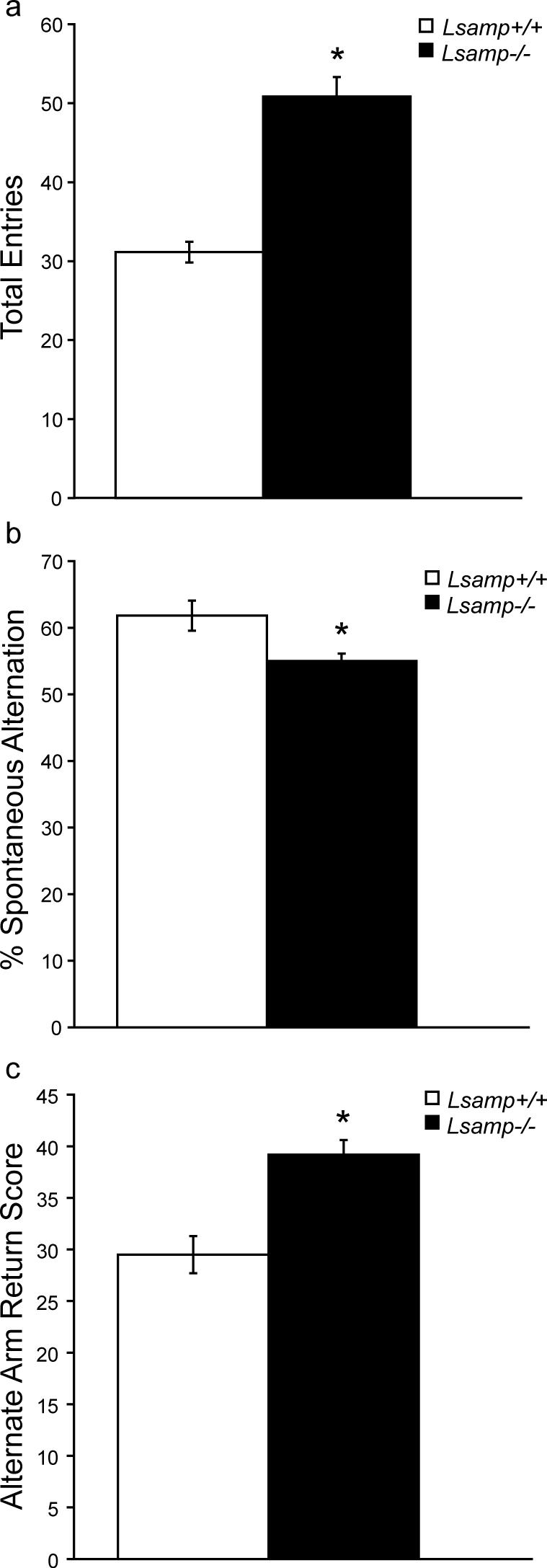
Y-maze data are presented in Figure 7. There was no main effect of sex on the y-maze measurements, so data were combined across sex for the analyses. There was a main effect of genotype on y-maze measurements (F(4,49)=17.70, p < 0.0001). As expected, Lsamp −/− mice were hyperactive during the 8-minute exposure to the novel y-maze, as measured by a significant increase (F(1,52)=53.43, p < 0.0001) in the total number of arm entries (Fig. 7a). Lsamp −/− mice also demonstrated a small, but significant decrease (F(1,52)=7.27, p = 0.011) in the percent spontaneous alternation (Fig. 7b). The deficit in spontaneous alternation was accounted for by an increase (F(1,52)=17.44, p = 0.0001) in alternate arm returns (Fig. 7c). There is no change in the proportion of same arm returns (data not shown).
Figure 7. Spontaneous alternation by Lsamp−/− mice in the Y-Maze.
The Lsamp−/− mice exhibit hyperactivity in the y-maze (a). There was a modest, but statistically significant decrease in spontaneous alternation in null mice (b), and an increase in alternate arm returns (c). (n=27−29/group)*p<0.05
4. Discussion
We found no evidence of gross changes in brain organization or connectivity in Lsamp −/− mice. This differs from previously reported findings using in vitro experimental paradigms in which LAMP mediated axon targeting and growth [24,55]. The current analyses are more consistent with a role for LAMP mediating finely specialized aspects of circuit formation and maturation in regions of the limbic system. LAMP, a cell-surface molecule, is not a receptor for any known neurotransmitter or neuromodulator, consistent with the hypothesis that altered expression of the protein is likely to lead to differences in connectivity rather than direct changes in traditional neurotransmitter signaling. There are wide-ranging developmental effects of deletion of axon guidance and cell adhesion molecules, resulting in very subtle to gross changes [3,47,52]. Cell adhesion molecules such as L1, NCAM, neurexins/neuroligins, and ephBs/ephrins are involved in the regulation of synapse formation and stability [12]. Deletion of these genes in model systems tends to result in more subtle defects that are consistent with most neuropathology found in psychiatric disorders, in which only modest changes at the cellular level (e.g. spine density, neuropil size, synaptic density) have been discerned [30]. Thus, it is possible that for brain regions in which LAMP is expressed, neuronal signaling is only subtly disturbed, but may lead to measurable changes in functional output of the circuits which are disrupted. Accordingly, the initial analysis of the Lsamp −/− mice demonstrates alterations of certain behaviors that relate to emotional reactivity in novel situations, without disruption of the development or maintenance of basic sensory and motor behaviors. This is reflected both by the normal developmental timing of sensorimotor responses and normal adult auditory startle and sensorimotor gating as measured by pre-pulse inhibition.
4.1 Select Changes in Behavior in Lsamp −/− Mice
The behavior of mice in a novel environment reflects a balance between the desire to explore (motor and behavioral activation) and fear (motor and behavioral inhibition) [8]. In three of the behavioral tasks that we used, both male and female Lsamp −/− mice demonstrated heightened behavioral activation as measured by their activity in a novel apparatus (distance in the activity chamber, entries on the EPM and entries in the y-maze). Lsamp−/− mice displayed hyperactivity when first placed in an open field activity chamber, but habituated to the same baseline activity levels as their Lsamp+/+ littermates over time. This is consistent with our hypothesis that the Lsamp −/− mice are not generally hyperactive, but rather are hyper-responsive to different novel environments. Increased locomotor activity as a response to a novel, stressful environment could be interpreted as increased behavioral activation, exploratory drive or as an inability to properly inhibit behavior in a threatening situation [9]. Although these domains are likely to be linked both behaviorally and neurobiologically, our data suggest that in the absence of Lsamp, mice are at least exhibiting heightened behavioral activation. During the first 10 minutes of the test, mice of both genotypes demonstrate increased activity above baseline, reflecting genotype-independent increased exploratory behavior during this time period. Male and female Lsamp−/− mice, however, have a heightened level of activity compared to their Lsamp+/+ littermates even during this portion of the test, indicating an increase in behavioral activation.
Increased activity also was evident in the EPM test. Lsamp−/− mice made a greater number of total arm entries, with a small increase in closed entries, exhibiting hyperactivity in the 5-minute exposure to a novel environment. Mice generally show a strong preference for the closed arms as measured by entries [19], which was evident in the analyses of the Lsamp+/+ mice. However, Lsamp−/− mice showed no preference in entries for either open or closed arms. One reasonable interpretation of the greater proportion of open entries, even in the context of the overall increase in entries, is a reduction in anxious behavior [37]. Alternatively, if the Lsamp−/− mice have an increased arousal state in response to stress, a lack of preference for either arm may reflect reduced behavioral inhibition and/or increased activation. Lsamp−/− mice also spend significantly more time in the open arms, which also is generally viewed as reflecting reduced anxiety. This conclusion follows from an ethological interpretation of the test and from the results of pharmacological manipulations, in which mice receiving anxiolytic drugs increase open arm time with a corresponding decrease in closed arm time, whereas anxiogenic drugs induce the opposite behaviors [37]. We propose an alternative hypothesis, one in which interpreting the EPM data as reflecting an altered anxiety state in the Lsamp−/− mice may not be accurate. For example, the increase in open arm time is accounted for by a significant decrease in time spent in the center of the maze. When Lsamp+/+ mice do spend time in the center, they generally inhibit motor behavior, remaining still, and appear to be “sizing up” the open arms prior to deciding which area to enter. In contrast, the Lsamp−/− mice do not inhibit their movement and, therefore, enter the open arms of the maze much more frequently than the Lsamp+/+ mice. There is precedence for this view, as the Ts65Dn mutant mouse also demonstrates greatly increased motor output with corresponding anxiolytic-like EPM results. Because of the hyperactivity, however, these changes have been interpreted not as anxiety, but as a lack of behavioral inhibition or reduced attention to environmental stimuli [7,33]. While recognizing that a simple explanation would involve primary disruption of the anxiety state, we suggest that Lsamp−/−mice exhibit heightened reactivity to stressful stimuli, revealed by hyper-activation and lack of appropriate behavioral inhibition.
Consistent with this view, heightened reactivity in the Lsamp−/− mice is evident in other behaviors, such as unprotected head-dips. Although there is a sex difference in degree of increase of unprotected head-dips, both male and female Lsamp−/− mice demonstrate increased risk assessment. Based on reports in the literature, the co-segregation of increased unprotected head-dips and open arm exploration in the Lsamp−/− mice is unusual. For example, factor analyses of mouse and rat behavior in the EPM revealed that increased head-dips load on the same factor as the traditional measures of anxiety, but is inversely correlated with open time and entries [10,46]. In one study [46], when protected and unprotected head-dips were analyzed separately, protected head-dips loaded on the anxiety factor, while unprotected head-dips did not fall into the categories discovered. Lsamp−/− mice do not show reduced head-dips in the protected portion of the maze, which would be associated with decreased anxiety. Instead, the increase in head-dips on the open arms of the EPM may be due to a combination of the increased time spent in the open arms of the maze and increased behavioral activation in open, more anxiety provoking areas of the maze. This interpretation again is consistent with the hypothesis that Lsamp−/− mice may experience behavioral hyper-activation or disinhibition in stressful environments. To gain further understanding and clarification of the underlying neurodevelopmental and molecular basis for these behavioral changes our laboratory is examining the integrity of the neural regulatory systems in which LAMP is expressed.
Spontaneous alternation in the y-maze takes advantage of the exploratory drive of rodents [28], in which animals typically investigate the newest area in an environment. The interpretation of deficits in alternation behavior can be complex because quantitative differences in the pattern of exploration have been interpreted in other studies as a reflection of decreased attention, deficits in short-term memory, changes in arousal or anxiety [23,28]. Along with their characteristic hyperactivity, Lsamp−/− mice exhibit a significant, though very modest decreased level of spontaneous alternation during the period when novelty-induced hyperactivity peaks. It is unlikely that this indicates altered anxiety in the traditional sense, because such measures of anxiety are decreased in the Lsamp−/− mice. Instead, we suggest that the altered performance on the maze may be due to a disrupted state of arousal or deficits in working memory. Consistent with the interpretation of altered arousal is the finding that Lsamp−/− mice displayed heightened reactivity in other novel environments. However, in order to definitively address the underlying cause of spontaneous alternation deficits, including the possibility of deficits in working memory, further detailed testing will be required.
Taken together, our data suggest that there is a complex behavioral deficit caused by the targeted deletion of the Lsamp gene. Rather than a primary defect in the regulation of anxiety state, we hypothesize that the mutation results in heightened and possibly maladaptive response to novel environmental stressors. This interpretation of the animal model experiments is consistent with the recent human genetic studies in which a polymorphism in the human Lsamp gene is associated with panic disorder in certain environments [26,32]. Lsamp is expressed robustly throughout the limbic circuitry responsible for mediating an animal's behavioral response to novelty (including circuitry that mediates fear, stress and exploratory behavior). Alterations in emotional regulation can be due both to direct changes in neurotransmitter function [16,22,50,53] and to alterations of synaptic connectivity [49,53]. Because Lsamp is expressed from the time that neurons become postmitotic prenatally and throughout the life of the animal, conditional and reversible manipulation of gene expression will be necessary to determine whether the behavioral dysfunction exhibited by the Lsamp−/− mice are due to differential development of limbic circuitry, direct modulation of mature synaptic function in the adult, or even both.
Acknowledgements
This research was supported by NIMH grant MH45507, the Vanderbilt Kennedy Center core grant NICHD HD15052. EH was a predoctoral fellow on NIH training grant T32 MH065215. We thank Dr. Jennifer Blackford of the Vanderbilt Kennedy Center Statistics and Methodology Core for important advice and Mrs. Pamela Cornuet for excellent technical assistance. We also thank Drs. Barbara Thompson, Gregg Stanwood and Kathie Eagleson for helpful comments during the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel FM, Brent R, Kingston RE, Moore DD, Sediman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; New York, NY: 1998. [Google Scholar]
- 3.Barallobre MJ, Del Rio JA, Alcantara S, Borrell V, Aguado F, Ruiz M, Carmona MA, Martin M, Fabre M, Yuste R, Tessier-Lavigne M, Soriano E. Aberrant development of hippocampal circuits and altered neural activity in netrin 1-deficient mice. Development. 2000;127:4797–810. doi: 10.1242/dev.127.22.4797. [DOI] [PubMed] [Google Scholar]
- 4.Broca P. Anatomie comparee des circonvolutions cerebrales: le grand lobe limbique. Rev. Anthropol. 1878;1:385–498. [Google Scholar]
- 5.Cote PY, Levitt P, Parent A. Distribution of limbic system-associated membrane protein immunoreactivity in primate basal ganglia. Neuroscience. 1995;69:71–81. doi: 10.1016/0306-4522(95)00185-l. [DOI] [PubMed] [Google Scholar]
- 6.Cote PY, Levitt P, Parent A. Limbic system-associated membrane protein (LAMP) in primate amygdala and hippocampus. Hippocampus. 1996;6:483–94. doi: 10.1002/(SICI)1098-1063(1996)6:5<483::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Coussons-Read ME, Crnic LS. Behavioral assessment of the Ts65Dn mouse, a model for Down syndrome: altered behavior in the elevated plus maze and open field. Behav Genet. 1996;26:7–13. doi: 10.1007/BF02361154. [DOI] [PubMed] [Google Scholar]
- 8.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 9.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 10.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 11.Dalgleish T. The emotional brain. Nature Reviews Neuroscience. 2004;5:582–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 12.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–20. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagleson KL, Pimenta AF, Burns MM, Fairfull LD, Cornuet PK, Zhang L, Levitt P. Distinct domains of the limbic system-associated membrane protein (LAMP) mediate discrete effects on neurite outgrowth. Molecular and Cellular Neuroscience. 2003;24:725–740. doi: 10.1016/s1044-7431(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 14.Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–41. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 15.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–52. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 16.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L. Molecular Probes of the Nervous System: Selected methods for antibody and nucleic acid prboes. Cold Spring Harbor Press; New York: 1993. [Google Scholar]
- 19.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 20.Horton HL, Levitt P. A unique membrane protein is expressed on early developing limbic system axons and cortical targets. Journal of Neuroscience. 1988;8:4653–61. doi: 10.1523/JNEUROSCI.08-12-04653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, Fan X, Song L, Riviere JB, Prevost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL,, Jr., McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–92. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- 22.Howell MP, Muglia LJ. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front Neuroendocrinol. 2006;27:275–84. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Keller F, Rimvall K, Barbe MF, Levitt P. A membrane glycoprotein associated with the limbic system mediates the formation of the septo-hippocampal pathway in vitro. Neuron. 1989;3:551–61. doi: 10.1016/0896-6273(89)90265-1. [DOI] [PubMed] [Google Scholar]
- 25.Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–3. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- 26.Koido K, Koks S, Must A, Reimets A, Maron E, Shlik J, Vasar V, Vasar E. Association analysis of limbic system-associated membrane protein gene polymorphisms in mood and anxiety disorders. European Neuropsychopharmacology. 2006;16:S9–S9. [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 29.Levitt P. A monoclonal antibody to limbic system neurons. Science. 1984;223:299–301. doi: 10.1126/science.6199842. [DOI] [PubMed] [Google Scholar]
- 30.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 31.Mann F, Zhukareva V, Pimenta A, Levitt P, Bolz J. Membrane-associated molecules guide limbic and nonlimbic thalamocortical projections. J Neurosci. 1998;18:9409–19. doi: 10.1523/JNEUROSCI.18-22-09409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron E, Koido K, Must A, Reimets A, Koks S, Vasar E, Vasar V, Shlik J. Association study of limbic system-associated membrane protein gene polymorphisms in panic disorder. European Neuropsychopharmacology. 2006;16:S459–S460. [Google Scholar]
- 33.Martinez-Cue C, Rueda N, Garcia E, Florez J. Anxiety and panic responses to a predator in male and female Ts65Dn mice, a model for Down syndrome. Genes Brain Behav. 2006;5:413–22. doi: 10.1111/j.1601-183X.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 34.Millan MJ. The neurobiology and control of anxious states. Progress in Neurobiology. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 35.Nelovkov A, Areda T, Innos J, Koks S, Vasar E. Rats displaying distinct exploratory activity also have different expression patterns of gamma-aminobutyric acid- and cholecystokinin-related genes in brain regions. Brain Res. 2006;1100:21–31. doi: 10.1016/j.brainres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Nelovkov A, Philips MA, Koks S, Vasar E. Rats with low exploratory activity in the elevated plus-maze have the increased expression of limbic system-associated membrane protein gene in the periaqueductal grey. Neurosci Lett. 2003;352:179–82. doi: 10.1016/j.neulet.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 37.Pellow S, File SE. Anxiolytic and Anxiogenic Drug Effects on Exploratory Activity in an Elevated Plus-Maze - a Novel Test of Anxiety in the Rat. Pharmacology Biochemistry and Behavior. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 38.Pimenta AF, Fischer I, Levitt P. cDNA cloning and structural analysis of the human limbic-system-associated membrane protein (LAMP) Gene. 1996;170:189–95. doi: 10.1016/0378-1119(96)84698-1. [DOI] [PubMed] [Google Scholar]
- 39.Pimenta AF, Reinoso BS, Levitt P. Expression of the mRNAs encoding the limbic system-associated membrane protein (LAMP): II. Fetal rat brain. Journal of Comparative Neurology. 1996;375:289–302. doi: 10.1002/(SICI)1096-9861(19961111)375:2<289::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Pimenta AF, Strick PL, Levitt P. Novel proteoglycan epitope expressed in functionally discrete patterns in primate cortical and subcortical regions. J Comp Neurol. 2001;430:369–88. [PubMed] [Google Scholar]
- 41.Pimenta AF, Zhukareva V, Barbe MF, Reinoso BS, Grimley C, Henzel W, Fischer I, Levitt P. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron. 1995;15:287–97. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 42.Prensa L, Gimenez-Amaya JM, Parent A. Chemical heterogeneity of the striosomal compartment in the human striatum. J Comp Neurol. 1999;413:603–18. [PubMed] [Google Scholar]
- 43.Prensa L, Richard S, Parent A. Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. J Comp Neurol. 2003;460:345–67. doi: 10.1002/cne.10627. [DOI] [PubMed] [Google Scholar]
- 44.Reinoso BS, Pimenta AF, Levitt P. Expression of the mRNAs encoding the limbic system-associated membrane protein (LAMP): I. Adult rat brain. Journal of Comparative Neurology. 1996;375:274–88. doi: 10.1002/(SICI)1096-9861(19961111)375:2<274::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Robertson RT, Mostamand F, Kageyama GH, Gallardo KA, Yu J. Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Brain Res Dev Brain Res. 1991;58:81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- 47.Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–80. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 49.Sandi C, Bisaz R. A model for the involvement of neural cell adhesion molecules in stress-related mood disorders. Neuroendocrinology. 2007;85:158–76. doi: 10.1159/000101535. [DOI] [PubMed] [Google Scholar]
- 50.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 51.Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–7. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- 52.Wiencken-Barger AE, Mavity-Hudson J, Bartsch U, Schachner M, Casagrande VA. The role of L1 in axon pathfinding and fasciculation. Cereb Cortex. 2004;14:121–31. doi: 10.1093/cercor/bhg110. [DOI] [PubMed] [Google Scholar]
- 53.Wood SJ, Toth M. Molecular pathways of anxiety revealed by knockout mice. Mol Neurobiol. 2001;23:101–19. doi: 10.1385/MN:23:2-3:101. [DOI] [PubMed] [Google Scholar]
- 54.Zacco A, Cooper V, Chantler PD, Fisher-Hyland S, Horton HL, Levitt P. Isolation, biochemical characterization and ultrastructural analysis of the limbic system-associated membrane protein (LAMP), a protein expressed by neurons comprising functional neural circuits. Journal of Neuroscience. 1990;10:73–90. doi: 10.1523/JNEUROSCI.10-01-00073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhukareva V, Levitt P. The limbic system-associated membrane protein (LAMP) selectively mediates interactions with specific central neuron populations. Development. 1995;121:1161–72. doi: 10.1242/dev.121.4.1161. [DOI] [PubMed] [Google Scholar]
- 56.Zhukareva VV, Chernevskaya N, Pimenta A, Nowycky M, Levitt P. Limbic System-Associated Membrane Protein (LAMP) Induces Neurite Outgrowth and Intracellular Ca2+ Increase in Primary Fetal Neurons. Mol Cell Neurosci. 1997;10:43–55. doi: 10.1006/mcne.1997.0639. [DOI] [PubMed] [Google Scholar]