Table 1.
Structure, anti-malarial activity and microsomal metabolism of 2-oxo-tetrahydro-1,8-naphthyridine-based PFTIs
| Compd. | X | R1 | R2 | Malaria PFT enzyme % inhibition at the indicated inhibitor concentration (nM) | ED50 for inhibition of parasite growth in vitro (nM)1 | Microsome metabolism half time (min)2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 50 | 5 | 0.5 | 3D7 | K1 | Naph | THQ | ||||
| 16 | Br | 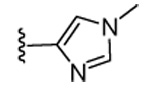 |
H | 74 | 28 | 0 | 0 | >5000 | >5000 | ND | ND |
| 17 | CN | 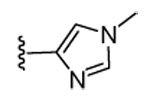 |
H | 94 | 66 | 32 | 15 | >5000 | >5000 | ND | ND |
| 18 | CN | 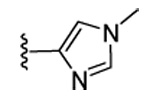 |
 |
99 | 98 | 80 | 27 | 420 | 300 | 52 | 15.6 |
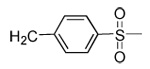
| 19 | CN | 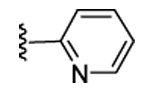 |
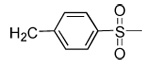 |
91 | 48 | 3 | 0 | 3100 | >5000 | 15 | ND |
| 20 | CN | 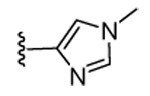 |
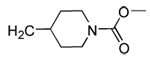 |
98 | 95 | 71 | 16 | 350 | 175 | >120 | 5.4 |
| 21 | CN | 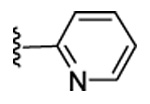 |
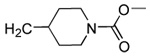 |
97 | 88 | 33 | 0 | 320 | 310 | ND | 3.8 |
ED50 is the concentration of compound that 50% inhibits the growth of parasites (chloroquine sensitive strain 3D7 or chloroquine resistant strain K1) in red blood cell cultures (measured according to ref. 6).
Given is the half-time for loss of parent compound when incubated with mouse liver microsomes according to the procedure given in ref. 6. Compounds tested are: 1) Column labeled Naph, the 2-oxo-tetrahydro-1,8-naphthyridines shown in the table; 2) Column labeled THQ, the corresponding tetrahydroquinoline-based PFT (analogs of 1) with the same R1 and R2 groups as the indicated 2-oxo-tetrahydro-1,8-naphthyridine. ND is not determined.
