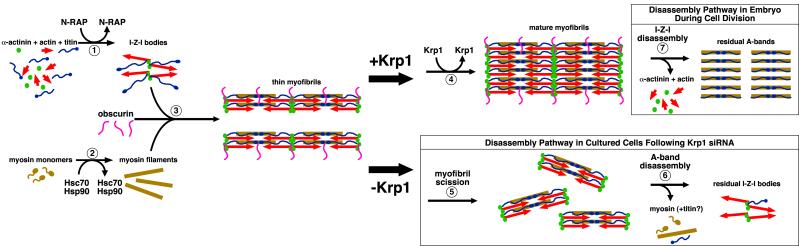
Figure 10.
A putative pathway for myofibril assembly (steps 1-4) highlighting the role of transiently associated proteins in organizing the major structural components. Disassembly pathways after Krp1 knockdown (steps 5-6) and during cell division in embryonic development (step 7) are also illustrated. (1) N-RAP promotes assembly of the I-Z-I structures containing actin, α-actinin, and N-terminal titin. (2) Myosin filaments form separately, with appropriate folding and assembly promoted by the Hsc70 and Hsp90 chaperone proteins. (3) Obscurin plays a role in promoting integration of the thick filaments with the I-Z-I structures, with titin associating with the myosin filaments along their length. This gives rise to thin myofibrils. (4) Finally, Krp1 promotes their lateral fusion to form mature myofibrils. (5-6) Krp1 knockdown results in scission of the thin myofibrils, followed by disassembly of the A-bands. The last organized structures observed contain α-actinin and actin, but not myosin. (7) In contrast, disassembly during cell division occurs by removal of α-actinin and actin, leaving organized A-bands. Subsequent A-band disassembly is not shown. See text for details.