Figure 2. αIIbβ3-dependent ERK dephosphorylation is not due to reduction in MEK1 kinase activity.
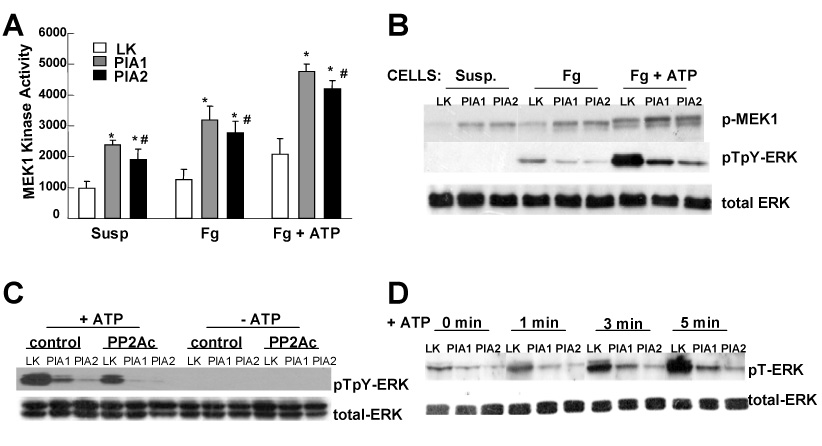
(A) Starved cells were plated on fibrinogen coated dishes (Fg, Fg + ATP) or left in suspension (Susp). After treatment with or without 40 µM ATP (Fg + ATP, Fg) for 5 min, cells were washed, MEK1 kinase activity quantified. Results are representative of three independent experiments. Presented data are the mean ± SD of triplicates. (*) P-value < 0.03 for PlA1 vs LK and PlA2 vs LK. (#) insignificant P-value for PlA1 vs PlA2. (B) WB analysis for the same cell extracts as described in (A) for the active forms of ERK (pTpY-ERK) and MEK1 (p-MEK1). (C) Cells were transfected with vectors containing a cDNA encoding for PP2A catalytic subunit (PP2Ac), or vector only (control). Twelve hours after transfection, cells were starved for 16 hours, and then plated on fibrinogen coated plates, and stimulated with 40 µM ATP (+ATP). WB was performed using antibody against active ERK (pTpY-ERK) or total ERK protein (total-ERK) as control. (D) Serum-starved cells were plated on fibrinogen, and stimulated with 40 µM ATP for the indicated time. Phosphorylation level on the Thr 183 residue of endogenous ERK was measured using an antibody specific to ERK phosphorylated at Thr 183 (pT-ERK). In both C and D, the results are representative of three independent experiments.
