Abstract
Predicting how populations respond to climate change requires an understanding of whether individuals or cohorts within populations vary in their response to climate variation. We used mixed-effects models on a song sparrow (Melospiza melodia) population in British Columbia, Canada, to examine differences among females and cohorts in their average breeding date and breeding date plasticity in response to the El Niño Southern Oscillation. Climatic variables, age and population density were strong predictors of timing of breeding, but we also found considerable variation among individual females and cohorts. Within cohorts, females differed markedly in their breeding date and cohorts also differed in their average breeding date and breeding date plasticity. The plasticity of a cohort appeared to be due primarily to an interaction between the environmental conditions (climate and density) experienced at different ages rather than innate inter-cohort differences. Cohorts that expressed higher plasticity in breeding date experienced warmer El Niño springs in their second or third breeding season, suggesting that prior experience affects how well individuals responded to abnormal climatic conditions. Cohorts born into lower density populations also expressed higher plasticity in breeding date. Interactions between age, experience and environmental conditions have been reported previously for long-lived taxa. Our current results indicate that similar effects operate in a short-lived, temperate songbird.
Keywords: breeding date, climate change, cohort variation, El Niño Southern Oscillation, mixed models, song sparrow
1. Introduction
Large-scale climatic variation affects population dynamics in a range of taxa (Post & Forchhammer 2002; Walther et al. 2002; Anders & Post 2006). In birds, one of the most commonly observed effects of climate are changes in the onset of breeding. Several North American species including Mexican jays (Aphelocoma ultramarina; Brown et al. 1999) and red-cockaded woodpeckers (Picoides borealis; Schiegg et al. 2002) have advanced their breeding dates in response to warming trends over the past few decades. Annual variation in the onset of breeding has also been linked to natural climatic oscillations such as the North Atlantic Oscillation in Europe (Forchhammer et al. 1998; Sanz 2003) and the El Niño Southern Oscillation (ENSO) in the Americas (Morrison & Bolger 2002; Wilson & Arcese 2003). The probable mechanisms behind annual shifts in breeding date include changes in the phenology of key food resources and effects of temperature on thermoregulatory costs and reproductive allocation by females (Arcese & Smith 1988; Brown et al. 1999; Morrison & Bolger 2002).
Recent studies have shown that individuals differ in their timing of breeding in the average environment (estimated as the intercept of the linear relationship between breeding date and climate) or their phenotypic plasticity in response to annual variation in climate (estimated as the slope between breeding date and climate). For example, in great tits (Parus major), collared flycatchers (Ficedula albicollis) and red deer (Cervus elaphus), individuals varied in both their intercept and slope terms, and those that bred earlier and showed higher plasticity typically had higher lifetime fitness (Brommer et al. 2005; Nussey et al. 2005a,b; see also Reed et al. 2006).
It is also possible that the cohorts within a population might differ in their response to variation in climate (Lindström 1999; Gaillard et al. 2003; Benton et al. 2006). Maternal effects or conditions experienced during the early development of a cohort can have lasting effects on future reproduction or survival (Albon et al. 1987; Forchhammer et al. 2001; Reid et al. 2003a). Environmental conditions experienced later in development may also influence breeding success. Reproductive performance often increases after the first breeding season (Martin 1995), and cohorts that experience an extreme climatic event in their second or third season may be better able to respond to those conditions than a cohort breeding for the first time. Other factors such as population density during that cohort's lifetime might also influence reproduction (Arcese et al. 1992). Identifying how cohorts within a population differ in their response to climate change is crucial for predicting how populations might respond to extreme climatic events or a long-term change in average conditions (Coulson et al. 2001; Lindström & Kokko 2002; Benton et al. 2006).
We used a 32-year study of song sparrows (Melospiza melodia) on Mandarte Island, British Columbia, to investigate within-population variation in the response of breeding date to annual fluctuations in climate. Using mixed-effects models, we first examined whether cohorts and individual females (nested within cohorts) differed in their mean response (intercept) and plasticity (slope) related to the ENSO. We then tested whether the intercept or slopes of cohorts and females affected their reproductive output. Finally, we examined whether variation in the slope of cohorts was related to population density or the climatic conditions that cohort experienced at different ages. Such analyses have been restricted to particular taxa (e.g. ungulates, seabirds) whose long lifespans and high philopatry allow study on how lifetime conditions affect a cohort's demographic response. Our study is one of the first to extend these analyses to a temperate songbird, a group for which these questions have been difficult to answer owing to their short lifespans and strong propensity to disperse from natal sites.
2. Material and methods
(a) Study population and field methods
The song sparrow population on Mandarte Island has been studied from 1975 to 2006. The size of the population was estimated from the number of breeding individuals in the last two weeks of April each year. Because all individuals in the study are colour-marked and the island is small (6 ha), we are confident that these spring counts had little or no error. Mark–recapture analyses implemented in Mark v. 5.1 (White & Burnham 1999) estimate our resighting probability to be 0.998. The annual number of breeding females has varied from 4 to 71 birds with an average of 35. Throughout the breeding period, individual territories were monitored every 3–5 days to identify the breeding pair and locate all nests. The date of first egg for each female was obtained by observing nests during laying or backdating from the date of hatch. For each nest, we also determined the clutch size and number of young that fledged and reached independence. We excluded nesting attempts from females that were given supplemental food during experimental studies in 1979 and 1985 (Arcese & Smith 1988). Further detail on the study population can be found in Smith et al. (2006).
(b) Mean response and plasticity of breeding date
To examine whether females and cohorts differ in the intercept or slope of their breeding date–ENSO relationship, we followed the approach of Pinheiro & Bates (2004; see also Brommer et al. 2005). In brief, this involved finding the top linear model with only fixed effects using maximum likelihood and then adding random effects to the top fixed-effects model using restricted maximum likelihood (REML). For these analyses, we used linear and linear mixed-effects models in R (R Core Development Team 2006). Based on our prior knowledge from this population (Wilson & Arcese 2003; Smith et al. 2006), we considered a set of nine fixed-effects-only models that included the Southern Oscillation Index (SOI), average daily temperature, total precipitation, age class and female density. Climate variables were the average monthly SOI, average daily temperature and total precipitation from January through April. Large-scale climatic indices such as the SOI may provide a better measure of overall climatic conditions than local weather and operate over larger temporal and spatial scales (Stenseth et al. 2003). However, because local temperature and precipitation might be important, we considered these as well and then estimated plasticity based on the climate variable that was most influential on breeding date. We first compared an intercept-only model with models including female density and age class. Age class (equal to or more than 1 year old) was included as a categorical effect because first-year females tend to breed later than older females (Nol & Smith 1987). We chose not to include specific ages because sample sizes of older age classes are small. To the top model containing these variables, we added SOI, temperature and precipitation separately. SOI values indicate sea-level pressure in the South Pacific Ocean and were obtained from the Climate Prediction Center of the National Oceanic and Atmospheric Association (http://www.cpc.ncep.noaa.gov/data/indices/soi). Low negative and high positive values of the SOI indicate El Niño and La Niña conditions, respectively. Temperature and precipitation data were obtained from the National Climate Archive of Environment Canada (http://climate.weatheroffice.ec.gc.ca/) for the Victoria International Airport station approximately 5 km northwest of our study site. Since there are multiple observations from cohorts and individual females, we model these as random effects as described in the final mixed-model below. Female density and climate variables were included as continuous effects and were mean-centred (Pinheiro & Bates 2004). We used Akaike's information criterion for small samples to identify top models and the ΔAICc and Akaike weights (wi) to infer support for different candidate models (Burnham & Anderson 2002).
After identifying fixed effects, we constructed a multilevel mixed-effects model with female and cohort as random effects (female nested within cohort). Models were fit using REML. This approach only allows for comparison of models with the same fixed-effects structure (Pinheiro & Bates 2004); therefore, mixed models were built using the top fixed-effects model as described previously. Preliminary analyses revealed that female slope effects were difficult to identify because the number of breeding-year observations for most females was only one to four. Models that only included females with four or more breeding years indicated differences in the slope term (a model with slope had AICc values less than 2 units than the one without), but the CIs were wide and there were strong correlations with the female intercept term. Therefore, we ran only the analysis with the intercept term for individual, which allowed us to include all females. We first added a term for the cohort intercept and then female intercept nested within cohort. These terms estimated differences among cohorts or individuals when they bred in the average environment. We then added a slope term for cohort, which estimated how plastic a cohort's average breeding date was in relation to the SOI. The AICc values were again used to evaluate support for the addition of random terms by comparing the mixed-effects models with the top fixed-effects model. The best linear unbiased predictors (BLUPs) of the random cohort and female effects were then extracted from the top model for further analyses. Assumptions of within-group error and normality of the random effects were examined following the analysis (Pinheiro & Bates 2004). After random effects were extracted, we used general linear models to test how the intercept (from both cohort and individual) and slope (cohort only) terms were related to mean annual fledgling production.
(c) Causes of variation in phenotypic plasticity of breeding date
We used general linear models to test whether conditions at birth (density and SOI) influenced the response of cohorts to climate change. We believed that population density and SOI were good surrogates of overall environmental quality at birth, with low population densities and low values of the SOI (i.e. warmer springs) indicative of better natal conditions. Because population size tends to be temporally autocorrelated, cohorts born into low-density populations may also experience low densities for much of their lifetime. To account for this possibility, we considered lifetime density, which was weighted for the number of breeding individuals in a cohort in different years.
To test whether the plasticity of a cohort was related to the age at which it experienced particular climatic conditions, we regressed the slope of the breeding date–climate model against SOI values in that cohort's first, second and third breeding seasons. Our hypothesis was that previous breeding experience would be an asset in responding to shifts in climate and therefore cohorts that experienced an anomalous event after their first breeding season would be more responsive to variation in climate (i.e. have a steeper slope) compared with a cohort whose first breeding season occurred during an extreme year. We also included an intercept-only model, which if supported would indicate that none of the above variables were influential. For these analyses, we used the global model to check goodness of fit by calculating (deviance/degrees of freedom).
3. Results
(a) Mean response and plasticity of breeding date
To examine variation in the intercept and slope of the breeding date–SOI relationship, we used 941 observations from 458 females across 29 cohorts. The top fixed-effects model contained age class, female density and SOI (tables 1a and 2). Local temperature and precipitation were less influential predictors of breeding date than the SOI, although inclusion of these two variables also improved model support. Females in their first breeding attempt typically bred approximately 6 days later than more experienced females. Females also bred earlier at lower population densities and more negative values of the SOI (table 2). From the top model equation with more than 1-year-old females and mean values for the other predictors (tables 1a and 2), a 10% increase in the SOI would lead to an expected 2.17 d delay in the onset of breeding. All models with random effects had substantially more support than models with only fixed effects (table 1b). The top model contained a term for the female intercept and the cohort by SOI interaction, indicating differences among females and cohorts in their breeding date in the average environment as well as differences in the response of cohorts to annual fluctuations in climatic conditions (table 2).
Table 1.
Summary of model selection results for factors affecting the onset of breeding for song sparrows on Mandarte Island, British Columbia. (Ln(L) is the value of the maximized log-likelihood function, AICc is Akaike's information criterion for small samples, ΔAICc is the scaled AICc relative to the top model, k is the number of model parameters and wi are the Akaike weights that provide a measure of the relative support for each model. Age, age class (equal to or more than 1 year old); SOI, Southern Oscillation Index. All climate variables are values from January to April (average monthly SOI, average daily temperature and total precipitation). For the examination of fixed effects, nine candidate models were considered but only the top five are shown here. Random effects models were fit with restricted maximum likelihood (REML). ‘top fixed’ refers to model age+female density+SOI.)
| model | ln(L) | AICc | ΔAICc | k | wi |
|---|---|---|---|---|---|
| (a) fixed effects only | |||||
| age+female density+SOI | −3625.70 | 7259.45 | 0.00 | 4 | 1.00 |
| female density+SOI | −3655.89 | 7317.81 | 58.35 | 3 | 0.00 |
| age+SOI | −3665.92 | 7337.86 | 78.41 | 3 | 0.00 |
| age+female density+temperature | −3678.92 | 7365.98 | 106.53 | 4 | 0.00 |
| age+female density+precipitation | −3741.42 | 7490.88 | 231.43 | 4 | 0.00 |
| (b) top fixed-effects model with random effects | |||||
| top fixed+female in cohort×SOI | −3573.56 | 7163.27 | 0.00 | 8 | 1.00 |
| top fixed+cohort×SOI | −3590.81 | 7195.74 | 32.47 | 7 | 0.00 |
| top fixed+female in cohort | −3594.56 | 7201.21 | 37.94 | 6 | 0.00 |
| top fixed+cohort | −3606.19 | 7222.44 | 59.17 | 5 | 0.00 |
| top fixed | −3625.70 | 7259.45 | 96.18 | 4 | 0.00 |
Table 2.
Random effects and fixed effects estimated from the linear mixed-effect model on breeding date of song sparrows on Mandarte Island, British Columbia. (Estimates were taken from the top mixed model in table 1 (top fixed+female in cohort×SOI). The standard deviation and 95% CI are provided for the random effects, along with residual variance. Estimates of the coefficients and the associated CIs are provided for the fixed effects. The correlation between the cohort slope and intercept terms was −0.33.)
| term | random effects | |
|---|---|---|
| s.d. | 95% CI | |
| female intercept | 5.30 | 4.31, 6.51 |
| cohort intercept | 2.95 | 1.88, 4.64 |
| cohort slope | 2.30 | 1.57, 3.39 |
| residual | 9.24 | 8.67, 9.85 |
| fixed effects | ||
|---|---|---|
| coefficient | 95% CI | |
| intercept | 113.76 | 110.87, 116.85 |
| female density | 0.24 | 0.18, 0.31 |
| age class | −3.88 | −5.49, −2.27 |
| average SOI | 2.85 | 1.84, 3.86 |
Cohorts that bred earlier (i.e. had a lower intercept) tended to produce more young per year (mean fledglings per female, r2=0.13, n=29, p=0.054, βcohort int=−0.14±0.07 (s.e.)). We also observed a weak trend suggesting that cohorts with steeper slopes had higher annual reproductive output (mean fledglings per female per cohort, r2=0.08, n=29, p=0.14, βcohort slope=0.20±0.13). Females that had a low intercept produced more young over their lifetime than those with a high intercept (r2=0.05, n=459, p<0.001, βfemale int=−0.41±0.08). Based on the regression equation for females, individuals with the earliest breeding dates would, over their lifetime, produce approximately 3.5 more fledglings than females with average breeding dates and approximately 8.5 more fledglings than females with the latest breeding dates.
(b) Causes of variation in phenotypic plasticity of breeding date
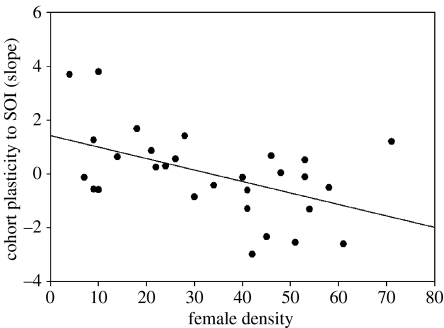
We used a linear model to examine how a cohort's slope was influenced by female density at birth and over their lifetime, and the January to April values of the SOI for their natal, first, second and third breeding years. As expected, we observed a positive correlation between the density at birth and the average density over the lifetime of the cohort (r=0.47). Female density and SOI in the birth year showed a weak negative correlation (r=−0.33), while correlations between all other variables were low (less than 0.30). The top model contained female density at birth and the SOI values in a cohort's second and third breeding seasons (table 3). The latter two variables were also in the three top models, which had a combined model weight of 0.971. Parameter estimates for the three variables from the top model (estimate±s.e.) were as follows: βbirth density=−0.032±0.015; βSOI 2nd=−0.70±0.27; βSOI 3rd=−0.47±0.27. The SOI in a cohort's birth year and first breeding year was not included in top models. Although a cohort's birth density and average lifetime density were correlated, models that included lifetime density had less support. Overall, the best-supported model suggested that cohorts with the highest plasticity estimate were those born at lower densities (figure 1) and those that experienced strong El Niño events in their second and third breeding seasons (figure 2).
Table 3.
Model selection results for the examination of cohort slope in relation to birth and lifetime density, and climatic conditions during the cohort's birth year, and first, second and third breeding seasons (averaged SOI values from January through April). (Global indicates the full model. Description of column headings is the same as given in table legend 1. Ten candidate models were considered (including a null model); wi values for models not shown were less than 0.01.)
| model | ln(L) | AICc | ΔAICc | k | wi |
|---|---|---|---|---|---|
| birth density+SOI 2nd+SOI 3rd | −37.83 | 83.70 | 0.00 | 4 | 0.69 |
| SOI 2nd+SOI 3rd | −40.22 | 86.47 | 2.08 | 3 | 0.24 |
| global | −35.97 | 86.06 | 5.95 | 7 | 0.04 |
| birth density+SOI 3rd | −42.67 | 91.37 | 6.94 | 3 | 0.02 |
Figure 1.
Relationship between female density at birth and the plasticity (slope) of a given cohort's breeding date–Southern Oscillation Index (SOI) interaction for song sparrows on Mandarte Island, British Columbia between 1975 and 2006. Cohort slopes were extracted from the best linear unbiased predictors (BLUPs) of the random effects from the top linear mixed-effects model (table 1b). BLUP estimates for the sample are centred around mean zero (25 out of 29 cohorts had a positive slope for average breeding date regressed against the SOI).
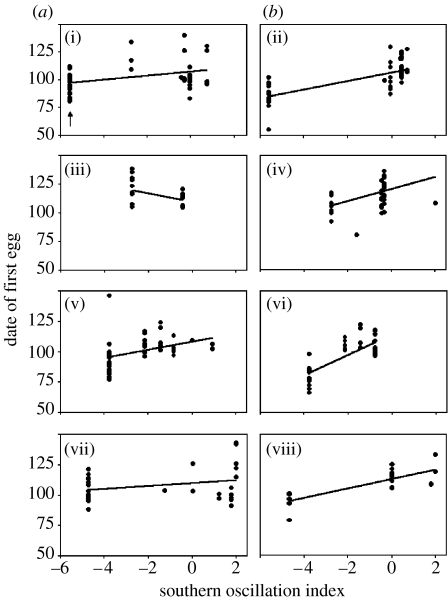
Figure 2.
Variation in breeding dates in relation to the SOI for cohorts that encountered the four strongest El Niño events during their (a) first or (b) second breeding season (1983 (i, ii), 1987 (iii, iv), 1992 (v, vi) and 1998 (vii, viii)). For each panel the leftmost series of observations represent the breeding dates for females in that cohort during the El Niño event (e.g. black arrow in (i)). Each circle in the panel indicates a breeding date for a female in the cohort that year. Low negative and high positive values of the SOI indicate El Niño and La Niña conditions, respectively. Date of first egg refers to Julian date beginning on 1 January.
4. Discussion
Our findings suggest that the ability of song sparrows to respond to variation in climate is partly dependent upon when individuals were born and the conditions cohorts experience at different ages. Climate and density are important predictors of breeding date in this population, with earlier breeding occurring under milder climates (e.g. El Niño years) and lower population densities (Arcese et al. 1992; Wilson & Arcese 2003). Food availability has a strong influence on breeding date (Arcese & Smith 1988) and it is probable that climate and density affect food resources through changes in phenology and competition, respectively. Warmer springs might also lower thermoregulatory costs of females and allow them to allocate more towards reproduction (Brown et al. 1999). Cohorts that encountered a warm El Niño spring in their second or third breeding season had steeper slopes than those experiencing an El Niño spring in their first season. This suggests that the estimate of a cohort's plasticity was affected more by interactions between age and environmental conditions than by innate differences among cohorts in the ability to respond to climate change. It further indicates that prior experience is beneficial in coping with abnormal climatic conditions.
Improved reproductive performance with age has been observed for both mammalian (Sydeman et al. 1991; Gaillard et al. 1992) and avian species (Martin 1995; Reid et al. 2003b), including song sparrows (Nol & Smith 1987). Our results show that age-related variation in performance may also be exaggerated in severe climates. Periods of food scarcity led to relatively lower reproductive success for inexperienced breeders in Tengmalm's owls (Aegolius funereus; Laaksonen et al. 2002) and Australasian gannets (Morus serrator; Bunce et al. 2005). On Mandarte Island, experienced female song sparrows may be better able to acquire resources in spring and respond more quickly should conditions favour an advance in the optimal laying date. Age-related effects of climatic variation are often observed in ungulate populations and models suggest that the ability of populations to respond to climate can differ considerably depending on the population age and structure when those events occur (Coulson et al. 2001; Clutton-Brock & Coulson 2002). Our results suggest that similar dependencies may operate in temperate songbirds.
We also found that density in the birth year was linked to a cohort's response to variation in climate. Environmental conditions (e.g. density, weather, disease) and/or maternal effects have been found to influence future reproduction and survival in birds and mammals (Lindström 1999; Forchhammer et al. 2001). Female red deer (C. elaphus) born into low-density populations were more plastic in their calving date than those born at high density, potentially because higher densities at birth have lasting effects on condition (Nussey et al. 2005a). It is possible that song sparrows born at high density experience greater competition and are in poorer average condition, which may lower their ability to respond to climate variation. High densities and competition in the post-breeding and winter periods may also affect timing of breeding the following spring (Arcese 1989), and a delayed first attempt for a cohort would influence its slope estimate. Variation in the age at first breeding for red-billed chough (Pyrrhocorax pyrrhocorax) cohorts was primarily driven by population size at maturity, while natal climatic conditions had a greater influence on juvenile survival and breeding longevity (Reid et al. 2003a). Beckerman et al. (2003) also showed how conditions experienced at birth and maturity might interact to shape future reproductive performance for a cohort.
The song sparrow population on Mandarte Island experienced severe declines at approximately 10-year intervals in 1979, 1989 and 1998, each followed by gradual rebuilding (Smith et al. 2006). Because the ENSO also tends to fluctuate in a periodic manner (Allan et al. 1996), some caution is warranted when interpreting potential cause and effect relationships between demography, individual tactics and climate. Over our 32-year study, however, El Niño, La Niña and more average years occurred coincident with low and high population densities, and we found no indication of strong autocorrelation in the ENSO or a lagged influence of the ENSO on population density. Thus, we have no reason to expect that birth density and future climatic conditions were related mechanistically.
Within populations, certain individuals often breed earlier and/or are more plastic in their breeding date in response to climate variation (Brommer et al. 2005; Nussey et al. 2005a,b). Our study revealed that within cohorts, females also differed markedly in their timing of breeding in the average environment and those that bred earlier fledged more young over their lifetime. Females that breed early may have higher reproductive success because they can initiate more nesting attempts per season (Wilson & Arcese 2003) and are less susceptible to brood parasitism from brown-headed cowbirds (Molothrus ater; Smith & Arcese 1994). Females that breed early may also be of higher quality overall and better able to successfully raise young. Cohorts that had earlier average breeding dates also tended to produce more young as expected given that females within those cohorts often bred earlier. Unfortunately, we were unable to examine differences in plasticity among females because most had only two to three breeding observations, which makes it difficult to estimate individual slopes. Analyses that considered females with four or more breeding years found that females had different slopes, but strong correlations with the intercept prevented meaningful comparisons. Other studies on collared flycatchers and great tits have found individual variation in response to climate, with more plastic individuals experiencing higher lifetime fitness (Brommer et al. 2005; Nussey et al. 2005b). By contrast, Reed et al. (2006) found no evidence for differences in plasticity in common guillemots (Uria aalge), perhaps because there is selection for synchrony among colonial breeders.
There is increasing evidence that population dynamics are influenced by large-scale climatic variation (Post & Forchhammer 2002; Anders & Post 2006) but not all populations respond equally, perhaps due to different life-history strategies or population structure (Both & Visser 2001; Coulson et al. 2001). With temperatures and weather variability expected to continue rising due to global warming (IPCC 2007), it is essential to understand what features of a population affect its response to climate variation. We found that the population density and climatic conditions experienced by a cohort at different ages affected its response to climate change. Cohorts may not differ inherently in their ability to respond to climate change, but those that encounter anomalous events in their second or third season are better able to adjust to those conditions resulting in a higher measure of plasticity. Our study provides one of the first examples that population-level responses of temperate songbirds may include nonlinear effects of climate and density on timing of breeding in different age groups, as noted previously for long-lived mammals (Coulson et al. 2001).
Acknowledgments
This work was conducted under permits of the UBC Animal Care Committee (A04-0177) and Environment Canada.
We are grateful to the many individuals who have contributed to the Mandarte project over the years, particularly W. Hochachka, L. F. Keller, A. B. Marr, J. M. Reid and the late J. N. M. Smith. We also thank D. H. Nussey, J. M. Gaillard, J. Wiess and M. C. Drever for their helpful comments on an earlier version of this manuscript and statistical analyses. The Tsawout and Tseycum First Nations bands kindly allowed us to work on Mandarte Island. Financial support for this research was provided by the National Science Foundation (P.A.), Natural Sciences and Engineering Research Council (S.W., D.R.N., A.G.W. and P.A.) and a Killam doctoral award to S.W.
References
- Albon S.D, Clutton-Brock T.H, Guinness F.E. Early development and population dynamics in red deer. II. Density independent effects and cohort variation. J. Anim. Ecol. 1987;56:69–81. doi:10.2307/4800 [Google Scholar]
- Allan R, Lindesay J, Parker D. CSIRO; Collingwood, Australia: 1996. El Niño Southern Oscillation and climate variability. [Google Scholar]
- Anders A.D, Post E. Distribution-wide effects of climate on population densities of a declining migratory landbird. J. Anim. Ecol. 2006;75:221–227. doi: 10.1111/j.1365-2656.2006.01034.x. doi:10.1111/j.1365-2656.2006.01034.x [DOI] [PubMed] [Google Scholar]
- Arcese P. Intrasexual competition and the mating system in primarily monogamous birds—the case of the song sparrow. Anim. Behav. 1989;38:96–111. doi:10.1016/S0003-3472(89)80069-7 [Google Scholar]
- Arcese P, Smith J.N.M. Effects of population density and supplemental food on reproduction in song sparrows. J. Anim. Ecol. 1988;57:119–136. doi:10.2307/4768 [Google Scholar]
- Arcese P, Smith J.N.M, Hochachka W.M, Rogers C.M, Ludwig D. Stability, regulation and the determination of abundance in an insular song sparrow population. Ecology. 1992;73:805–822. doi:10.2307/1940159 [Google Scholar]
- Beckerman A.P, Benton T.G, Lapsley C.T, Koesters N. Talkin' 'bout my generation: environmental variability and cohort effects. Am. Nat. 2003;162:754–767. doi: 10.1086/381056. doi:10.1086/381056 [DOI] [PubMed] [Google Scholar]
- Benton T.G, Plaistow S.J, Coulson T.N. Complex population dynamics and complex causation: devils, details and demography. Proc. R. Soc. B. 2006;273:1173–1181. doi: 10.1098/rspb.2006.3495. doi:10.1098/rspb.2006.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, Visser M.E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. doi:10.1038/35077063 [DOI] [PubMed] [Google Scholar]
- Brommer J.E, Merilä J, Sheldon B.C, Gustafsson L. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution. 2005;59:1362–1371. doi:10.1554/04-561 [PubMed] [Google Scholar]
- Brown J.L, Li S.H, Bhagabati N. Long-term trend toward earlier breeding in an American bird: a response to global warming. Proc. Natl Acad. Sci. USA. 1999;96:5565–5569. doi: 10.1073/pnas.96.10.5565. doi:10.1073/pnas.96.10.5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce A, Ward S.J, Norman F.I. Are age-related variations in breeding performance greatest when food availability is limited? J. Zool. 2005;266:163–169. doi:10.1017/S0952836905006734 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information-theoretic approach. [Google Scholar]
- Clutton-Brock T.H, Coulson T. Comparative ungulate dynamics: the devil is in the detail. Phil. Trans. R. Soc. B. 2002;357:1285–1288. doi: 10.1098/rstb.2002.1128. doi:10.1098/rstb.2002.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson T, Catchpole E.A, Albon S.D, Morgan B.J.T, Pemberton J.M, Clutton-Brock T.H, Crawley M.J, Grenfell B.T. Age, sex, density, winter weather and population crashes in Soay sheep. Science. 2001;292:1528–1531. doi: 10.1126/science.292.5521.1528. doi:10.1126/science.292.5521.1528 [DOI] [PubMed] [Google Scholar]
- Forchhammer M.C, Post E, Stenseth N.C. Breeding phenology and climate. Nature. 1998;391:29–30. doi:10.1038/34070 [Google Scholar]
- Forchhammer M.C, Clutton-Brock T.H, Lindström J, Albon S. Climate and population density induce long-term cohort variation in a northern ungulate. J. Anim. Ecol. 2001;70:721–729. doi:10.1046/j.0021-8790.2001.00532.x [Google Scholar]
- Gaillard J.M, Sempere A.J, Boutin J.M, Vanlaere G, Boisaubert B. Effects of age and body-weight on the proportion of females breeding in a population of roe deer (Capreolus capreolus) Can. J. Zool. 1992;70:1541–1545. [Google Scholar]
- Gaillard J.M, Loison A, Toïgo C, Delorme D, Van Laere G. Cohort effects and deer population dynamics. Ecoscience. 2003;10:412–420. [Google Scholar]
- IPCC. Climate change 2007: impacts, adaptation, and vulnerability. In: Parry M, Canziani O, Palutikof J, editors. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- Laaksonen T, Korpimäki E, Hakkarainen H. Interactive effects of parental age and environmental variation on the breeding performance of Tengmalm's Owls. J. Anim. Ecol. 2002;71:23–31. doi:10.1046/j.0021-8790.2001.00570.x [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. doi:10.1016/S0169-5347(99)01639-0 [DOI] [PubMed] [Google Scholar]
- Lindström J, Kokko H. Cohort effects and population dynamics. Ecol. Lett. 2002;5:338–344. doi:10.1046/j.1461-0248.2002.00317.x [Google Scholar]
- Martin K. Patterns and mechanisms for age-dependent reproduction and survival in birds. Am. Zool. 1995;35:340–348. [Google Scholar]
- Morrison S.A, Bolger D.T. Variation in a sparrow's reproductive success with rainfall: food and predator-mediated processes. Oecologia. 2002;133:315–324. doi: 10.1007/s00442-002-1040-3. doi:10.1007/s00442-002-1040-3 [DOI] [PubMed] [Google Scholar]
- Nol E, Smith J.N.M. Effects of age and breeding experience on seasonal reproductive success in the song sparrow. J. Anim. Ecol. 1987;56:301–313. doi:10.2307/4816 [Google Scholar]
- Nussey D.H, Clutton-Brock T.H, Elston D.A, Albon S.D, Kruuk L.E.B. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 2005a;74:387–396. doi:10.1111/j.1365-2656.2005.00941.x [Google Scholar]
- Nussey D.H, Postma E, Gienapp P, Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005b;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York, NY: 2004. Mixed effects models in S and S-plus. [Google Scholar]
- Post E, Forchhammer M.C. Synchronization of animal population dynamics by large-scale climate. Nature. 2002;420:168–171. doi: 10.1038/nature01064. doi:10.1038/nature01064 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2006 R: a language and environment for statistical computing, reference index, version 2.2.1. Vienna, Austria: R Foundation for Statistical Computing. (http://www.R-project.org)
- Reed T.E, Wanless S, Harris M.P, Frederikson M, Kruuk L.E.B, Cunningham E.J.A. Responding to environmental change: plastic responses vary little in a synchronous breeder. Proc. R. Soc. B. 2006;273:2713–2719. doi: 10.1098/rspb.2006.3631. doi:10.1098/rspb.2006.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Bignal E.M, Bignal S, McCracken D.I, Monaghan P. Environmental variability, life history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 2003a;72:36–46. doi:10.1046/j.1365-2656.2003.00673.x [Google Scholar]
- Reid J.M, Bignal E.M, Bignal S, McCracken D.I, Monaghan P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 2003b;72:765–776. doi:10.1046/j.1365-2656.2003.00750.x [Google Scholar]
- Sanz J.J. Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography. 2003;26:45–50. doi:10.1034/j.1600-0587.2003.03251.x [Google Scholar]
- Schiegg K, Pasinelli G, Walters J.R, Daniels S.J. Inbreeding and experience affect response to climate change in endangered woodpeckers. Proc. R. Soc. B. 2002;269:1153–1159. doi: 10.1098/rspb.2002.1966. doi:10.1098/rspb.2002.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.N.M, Arcese P. Brown-headed cowbirds and an island population of song sparrows: a 16 year study. Condor. 1994;96:916–934. doi:10.2307/1369102 [Google Scholar]
- Smith J.N.M, Keller L.F, Marr A.B, Arcese P. Oxford University Press; New York, NY: 2006. Conservation and biology of small populations: the song sparrows of Mandarte Island. [Google Scholar]
- Stenseth N.C, Ottersen G, Hurrell J.W, Mysterud A, Lima M, Chan K, Yoccoz N.G, Ådlansvik B. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. doi:10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydeman W.J, Huber H.R, Emslie S.D, Ribic C.A, Nur N. Age-specific weaning success of northern elephant seals in relation to previous breeding experience. Ecology. 1991;72:2204–2217. doi:10.2307/1941571 [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.F, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- White G.C, Burnham K.P. Program Mark: survival estimation from populations of marked animals. Bird Study. 1999;46:S120–S139. [Google Scholar]
- Wilson S, Arcese P. El Niño drives timing of breeding but not population growth in the song sparrow (Melospiza melodia) Proc. Natl Acad. Sci. USA. 2003;100:11 139–11 142. doi: 10.1073/pnas.1931407100. doi:10.1073/pnas.1931407100 [DOI] [PMC free article] [PubMed] [Google Scholar]