Figure 6.
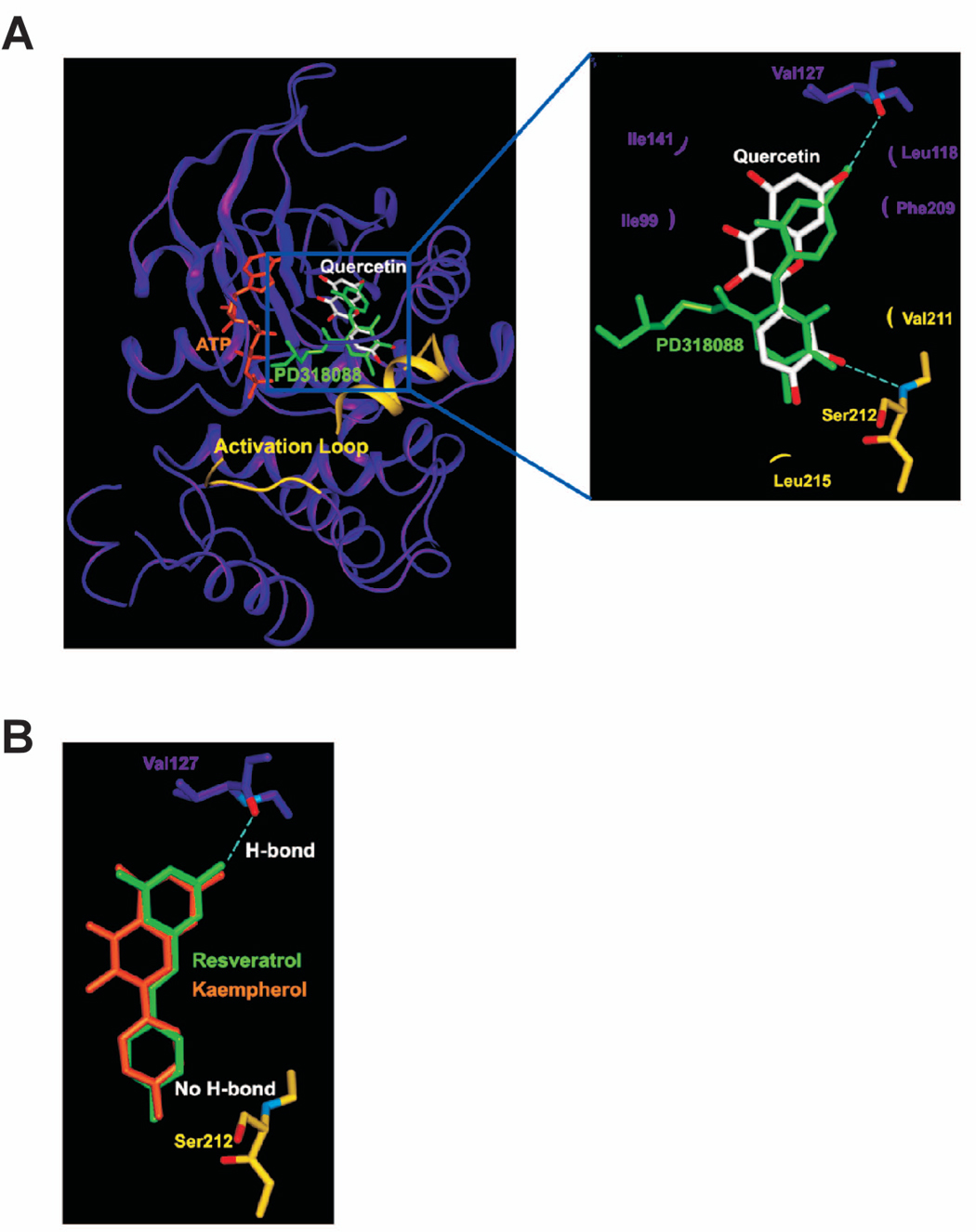
Modeling study of the MEK1 binding of quercetin, resveratrol, or kaempherol. A. Hypothetical model of MEK1-quercetin complex. Quercetin (white color) binds to the pocket adjacent to the ATP (orange) binding site. PD318088 (green) is superimposed on the model structure of MEK1-quercetin complex for comparison. The partially disordered activation loop is colored yellow. The residues involved in the interactions with quercetin are indicated. The hydrogen bonds are depicted as dashed lines. B. Hypothetical model of MEK1 in complex with resveratrol (green) or kaempherol (orange). Although each of these compounds can retain the hydrogen bond with Val127 and the van der Waals interactions involved in the binding of quercetin to MEK1, neither compound can form a hydrogen bond with the activation loop of MEK1 due to the lack of a hydrogen bond acceptor at the 3′ position of their respective ring adjacent to the activation loop.
