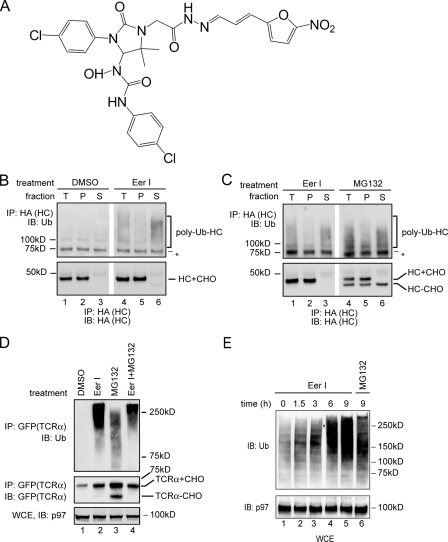
FIGURE 1.
Accumulation of polyubiquitinated proteins in EerI-treated cells. A, structure of EerI. B, EerI treatment leads to an accumulation of polyubiquitinated HC in cytosol. A9 cells were treated with either Me2SO (DMSO) or EerI (10 μm, 14 h), and permeabilized with the detergent digitonin. A portion of the cells was solubilized directly (T), whereas the rest of each sample was fractionated into a pellet (P) and a supernatant fraction (S). HC was immunoprecipitated (IP) with an anti-HA antibody under denaturing condition and analyzed by immunoblotting (IB). (HC+CHO, glycosylated HC; HC-CHO, deglycosylated HC; asterisk, a nonspecific protein cross-reacting with the ubiquitin antibody). C, as in B, except that A9 cells treated with either EerI or MG132 (10 μm) were analyzed. D, accumulation of polyubiquitinated TCRα in EerI-treated cells. Where as indicated, TCRα-GFP-expressing cells were treated with both EerI and MG132 (TCRα+CHO, glycosylated TCRα; TCRα-CHO, deglycosylated TCRα). A fraction of the extracts (WCE) was analyzed directly by immunoblotting. E, accumulation of polyubiquitinated proteins in EerI-treated cells. WCE of 293T cells treated with the indicated compounds were analyzed by immunoblotting.