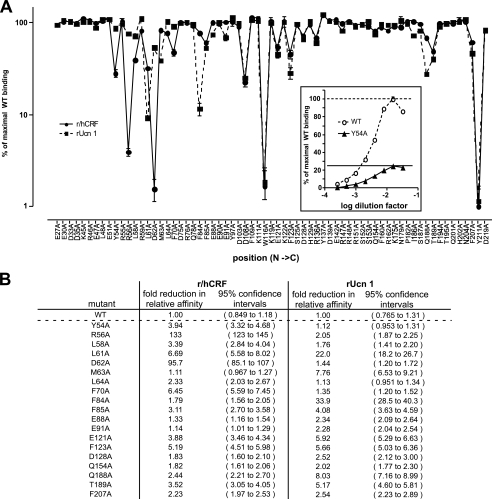
FIGURE 1.
Summary of the effect of alanine substitution of selected residues of human CRF-BP on maximum r/hCRF or rUcn 1 binding. Serial dilutions of each mutant were incubated with a fixed amount of 125I-[d-Tyr0]r/hCRF or 125I-[d-Tyr0]rUcn 1. The amount of bound radioligand increases with increasing concentrations of CRF-BP, until it reaches a maximum and starts to decrease with increasing CRF-BP concentration, when the capacity of the CRF-BP antiserum is no longer sufficient to capture all CRF-BP (inset). The maximum tracer binding capacity is an approximate indicator of affinity. For example (inset), CRF-BPY54A binds ∼25% of the tracer that is bound by WT CRF-BP, indicative of reduced affinity for r/hCRF. Using this method we determined the maximum tracer binding capacity for each alanine mutant in duplicate for independently expressed and purified CRF-BP preparations using r/hCRF and rUcn 1 tracers (A). We expressed these maxima relative to the maximal 125I-[d-Tyr0]r/hCRF (circles, solid line) and 125I-[d-Tyr0]rUcn 1 (boxes, dashed line) binding capacity of WT CRF-BP, which was defined as 100%. Alanine substitutions that affect the affinity for r/hCRF and/or rUcn 1 are characterized by a decrease in their percent of maximal binding. Changes in relative affinity were determined separately by competitive binding assays (B). Only mutants that affect affinity for either peptide by 2-fold or more are shown. Note that the affinities of CRF-BPW116A and CRF-BPY211A could not be determined as neither mutant binds detectable amounts of r/hCRF or rUcn 1 tracers. See supplemental Table 1 for a comprehensive list of the relative affinity for all mutants.