FIGURE 4.
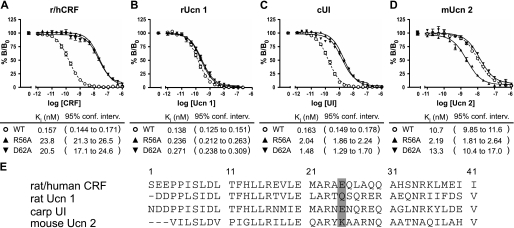
The direction and severity of the change in affinity for CRF family peptides correlates with the charge of the amino acid side chain at position 25 of the ligand. CRF-BPR56A and CRF-BPD62A have 100- and 10-fold reductions in affinity for r/hCRF (A) and carp urotensin I (C), respectively. Affinity for rUcn 1 is reduced by less than 2-fold (B). In contrast, CRF-BPR56A has increased affinity for mUcn 2, whereas alanine substitution of Asp62 has no effect on mUcn 2 affinity (D). Note that the reduced affinity of r/hCRF and cUI correlates with a glutamic acid at position 25, whereas mUcn 2 has a basic lysine at the equivalent position and displays increased affinity for CRF-BPR56A. The minor effects of either CRF-BP mutant on rUcn 1 affinity correspond with the absence of an organic base or acid in the side chain of amino acid position 25 (E). In all experiments 125I-[d-Tyr0]rUcn 1 was used as tracer. Ki values and 95% confidence intervals are derived from two or more separate experiments.