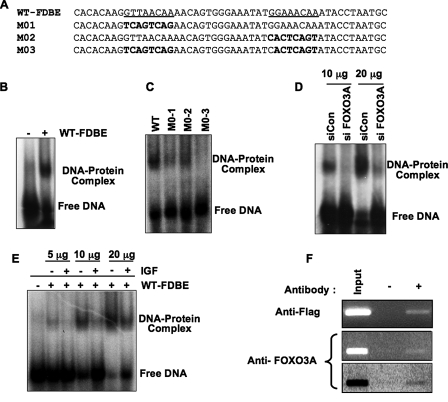
FIGURE 2.
FOXO3A binds its cognate sequences within the Prx III promoter. A and B, binding of FOXO3A to Prx III promoter in vitro. Representative EMSA demonstrating that a 50 bp double-stranded oligonucleotide encompassing both putative FOXO3A binding sites (sequence WT-FDBE in panel A) exhibits an electromobility shift when incubated with nuclear extracts from human cardiac fibroblasts (see under “Experimental Procedures” for EMSA). C, binding of FOXO3A to Prx III is specific to –267 and –259 sites. To show specificity for binding, the FOXO3A binding sites within Prx III promoter were replaced with random sequences (sequences M01, M02, and M03 as indicated in A) and used in EMSA performed on HEK-293 cells nuclear extracts. D, endogenous FOXO3A binds to Prx III promoter. To test whether endogenous FOXO3A is required, HEK-293 cells were transfected (as described under “Experimental Procedures”) with siCon or siFOXO3A, and 96 h later cells were harvested and processed, and the corresponding nuclear extracts (10 and 20 μg, respectively) were employed in mobility shift assay (D). Each blot is representative of three similar experiments. E, binding of FOXO3A to the Prx III promoter is impaired by IGF-1 treatment. HEK-293 cells were treated with IGF-1 (40 ng/ml medium) for 8 h and the corresponding nuclear extracts employed in EMSA as described under “Experimental Procedures.” Shown are increasing amounts of nuclear lysates. F, FOXO3A binds in vivo to Prx III promoter. Chromatin immunoprecipitation assays were performed in HEK-293 cells as described under “Experimental Procedures.” HEK-293 cells were transfected with control or FOXO3A plasmids and harvested for ChIP assay after 48 h. FOXO3A was immunoprecipitated either with an antibody against FLAG from cells transfected with pcDNA3-FLAG-FOXO3 (upper panel) or with FOXO3A antibody (middle and lower panels). This is representative of three similar experiments. The lower panel is identical with middle panel; however the image was inverted to enhance the intensity of FOXO3A PCR band.