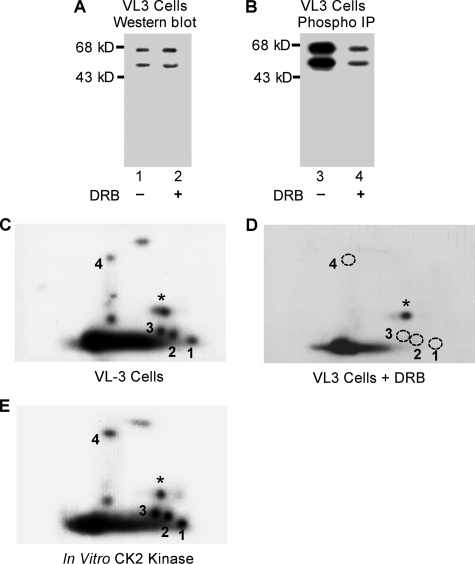
FIGURE 6.
Phosphorylation of the four novel sites is dependent on CK2 kinase activity. A, VL3-3M2 cells were treated with 50 μm DRB to inhibit CK2 activity, and Ikaros protein concentrations in treated and untreated cells were determined by Western blot. B, Ikaros proteins were immunoprecipitated (IP), resolved on SDS-PAGE gel, transferred to nitrocellulose membrane, and exposed to measure 32P incorporation. Two-dimensional phosphopeptide maps of Ikaros from untreated (C) or DRB-treated (D) VL3-3M2 cells were generated. Phosphopeptides representing novel phosphorylation sites are numbered 1–4. A star indicates a phosphopeptide representing a previously identified phosphorylation site (amino acid 63). The phosphopeptides that were not detected in VL3-3M2 cells after DRB treatment are indicated with a dashed circle. E, phosphopeptide mapping of an in vitro kinase assay with recombinant Ikaros and CK2 kinase.