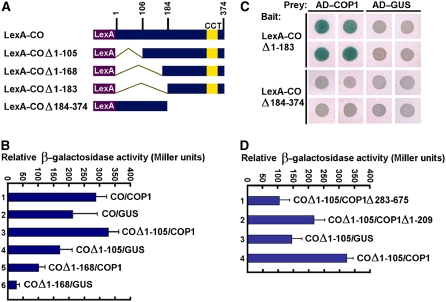
Figure 2.
Yeast Two-Hybrid Assay of Interaction between CO and COP1.
(A) Yeast two-hybrid bait constructs comprising CO fragments fused to the LexA DNA binding domain (LexA).
(B) Quantitative yeast two-hybrid assays define domains of CO essential for the interaction with COP1. All vector combinations are given as bait/prey. Standard deviations are indicated by error bars (n = 10). The bait constructs showed significant background (samples 2, 4, and 6). However, the β-galactosidase activity increased dramatically when the COΔ1-105 or COΔ1-168 bait was coexpressed with the COP1 prey (samples 3 and 5).
(C) Plate assays showing interaction between the CCT-containing domain of CO (COΔ1-183) and COP1. Blue precipitate represents cumulative β-galactosidase activity resulting from the activation of the lacZ reporter gene by protein–protein interaction. Quadruplicate yeast patches expressing the indicated LexA hybrid (rows) and the indicated AD hybrid (columns) were derived from four independent transformants.
(D) Quantitative yeast two-hybrid assays showing interaction of the C-terminal domain of COP1 with CO. COP1 Δ283-675 and COP1Δ1-209 indicate COP1 fragments containing the N-terminal RING finger and the C-terminal WD40 repeat domain, respectively.