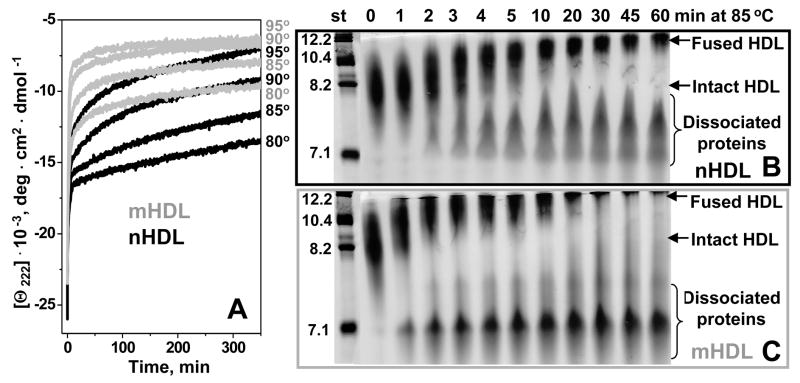
Figure 4.
Effect of mild oxidation by Cu2+ on the time course of HDL protein unfolding, protein dissociation and particle fusion. (A) HDL samples (2.5 mg/mL protein) that were non-oxidized (nHDL, black) or oxidized to stage 2 (mHDL, grey) were diluted to 33 μg/ml protein in buffer B containing 150 mM NaCl, and were subjected to T-jumps from 25 °C to 80, 85, 90, or 95 °C (shown on the lines). The time course of protein unfolding was monitored by CD at 222 nm. Similar samples of nHDL (B) or mHDL (C) containing 1 mg/mL protein were incubated at 85 °C, the aliquots were taken after 1–60 min of incubation and were subjected to non-denaturing gel electrophoresis to monitor the time course of protein dissociation and particle fusion. Lane numbers show incubation times (in min) at 85 °C; molecular size standards are shown in nm. Normal apoA-I and A-II are highly self-associated at these concentrations, and the proteins that gradually dissociate from HDL at high temperature run as oligomers 7–8 nm in size (B); self-association of mildly oxidized proteins (C) may be affected by their reduced hydrophobicity.