Figure 1.
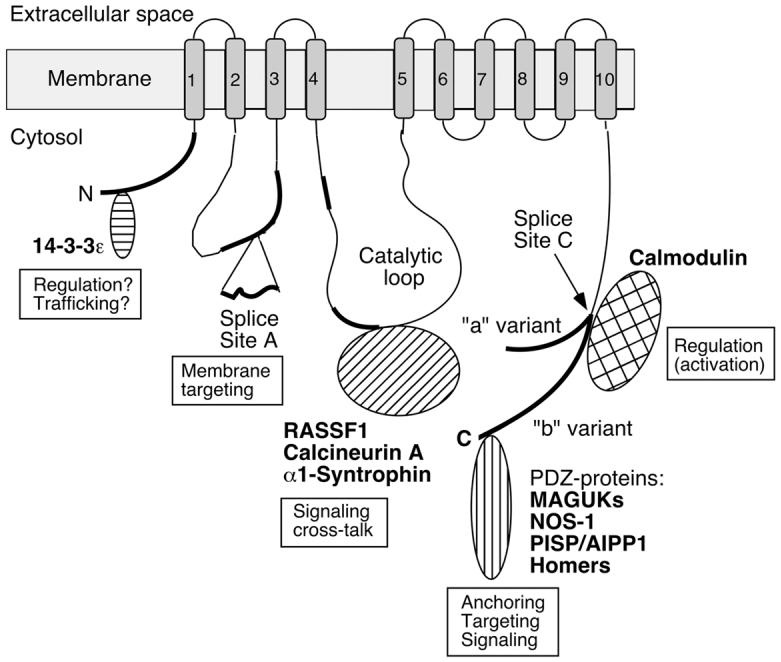
Scheme of the PMCA emphasizing regions of structural diversity among isoforms and sites of protein-protein interactions. Membrane-spanning regions are numbered 1-10 and shown as shaded boxes. The amino- (N) and carboxyl-terminal (C) ends are labeled, and the position of the large catalytic loop is indicated. Regions of significant sequence divergence among isoforms are shown as thick black lines. “Splice Site A” and “Splice Site C” denote the regions affected by alternative splicing. At site A the insertion of a peptide segment encoded by alternatively spliced exon(s) is indicated; at site C the two major splice variants (“a” and “b”) are shown with separate tails to indicate their divergent reading frames. Various PMCA-interacting proteins are schematically shown near the domain of the PMCA where they bind, and their known or suspected roles in providing functional diversity are indicated. The PMCA is represented in its activated state with calmodulin bound to the C-tail. For details, see the text.
