Abstract
Two known types of leptin-responsive neurons reside within the arcuate nucleus: the agouti gene-related peptide (AgRP)/neuropeptide Y (NPY) neuron and the proopiomelanocortin (POMC) neuron. By deleting the leptin receptor gene (Lepr) specifically in AgRP/NPY and/or POMC neurons of mice, we examined the several and combined contributions of these neurons to leptin action. Body weight and adiposity were increased by Lepr deletion from AgRP and POMC neurons individually, and simultaneous deletion in both neurons (A+P LEPR-KO mice) further increased these measures. Young (periweaning) A+P LEPR-KO mice exhibit hyperphagia and decreased energy expenditure, with increased weight gain, oxidative sparing of triglycerides, and increased fat accumulation. Interestingly, however, many of these abnormalities were attenuated in adult animals, and high doses of leptin partially suppress food intake in the A+P LEPR-KO mice. Although mildly hyperinsulinemic, the A+P LEPR-KO mice displayed normal glucose tolerance and fertility. Thus, AgRP/NPY and POMC neurons each play mandatory roles in aspects of leptin-regulated energy homeostasis, high leptin levels in adult mice mitigate the importance of leptin-responsiveness in these neurons for components of energy balance, suggesting the presence of other leptin-regulated pathways that partially compensate for the lack of leptin action on the POMC and AgRP/NPY neurons.
LEPTIN IS A circulating adipokine that is a critical physiological modulator of body mass, body composition, insulin sensitivity, and reproductive capacity (1). The distribution of leptin receptors within the hypothalamus is broad, and leptin causes widespread neuronal modulation (2). Loss of leptin signaling results in hyperphagia, primarily by increasing meal size (3,4). In addition, loss of leptin signaling results in decreased energy expenditure by decreasing core body temperature (5) and physical activity (6,7). Leptin’s modulation of various specific hypothalamic areas and neuronal types with respect to energy balance and neuroendocrine function are beginning to be critically assessed (8,9).
Two types of leptin-responsive neurons in the hypothalamic arcuate nucleus have received a great deal of attention, principally due to their demonstrated involvement in the melanocortin and neuropeptide Y (NPY) systems that modulate ingestive behavior and metabolism. The proopiomelanocortin (POMC)/cocaine amphetamine regulated transcript (CART) neuron is a crucial component of the metabolism neural network as ablation of the POMC gene in rodents (10) and humans (11) leads to hyperphagia and obesity. The loss of leptin receptors on POMC cells causes mild obesity and diminished POMC mRNA (9) and targeted ablation of POMC neurons leads to hyperphagia and obesity (12). The NPY/agouti gene related peptide (AGRP) neuron is another critical network component because it secretes two highly potent orexigenic peptides. The dependence of hyperphagia and obesity on NPY in leptin signaling deficient rodents has been reported, whereas AGRP overexpression (by transgenesis or direct intracranial injections) leads to hyperphagia and obesity (13). Additionally, leptin directly stimulates the expression of POMC in the arcuate nucleus (14) and leptin inhibits the transcription of the Npy and Agrp genes (15,16). Targeted deletion of NPY/AGRP neurons in adult mice produces hypophagia, although the same manipulation in neonates does not affect food intake or body weight (17,18). Destruction of both POMC/CART neurons and NPY/AGRP neurons in adult rodents produces relatively mild obesity and hyperphagia (12,19). Finally, viral mediated expression of leptin receptors into the arcuate nucleus of leptin receptor-deficient rodents ameliorated obesity, hyperglycemia, and locomotor activity (20,21).
We report on the developmental progression of phenotypes of mice with selective ablation of leptin receptor (LEPR)-B expression in AGRP/NPY and POMC/CART neurons with cyclization recombinase (CRE)-locus of crossing over technology. Based on our data from periweaning and adult mice, we propose that the rapid periweaning growth phase is characterized by the emergence of leptin-regulated control of ingestion and substrate use that is mediated by POMC and AGRP/NPY neurons. The adult maintenance phase is characterized by leptin-regulated substrate use that modulates body composition. Finally, the incomplete recapitulation of the leptin signaling deficient phenotype by loss of LEPR in these two neuronal types indicates that other biochemically defined neuronal types are critical to mediating the effects of leptin signaling.
Materials and Methods
Animals
The Agrp-CRE, POMC-CRE, Z/EG, and Lepr-flox/flox mice have been previously described (9,12,22,23). The Lepr-flox/flox mice were backcrossed to the FVB/NJ strain for six generations (N6), whereas the CRE transgenic lines were coisogenic lines. Agrp-CRE Lepr-flox/flox and Pomc-CRE Lepr-flox/flox mice were crossed to obtain Agrp-CRE + Pomc-CRE Lepr-flox/flox mice. All mice used for subsequent studies were generated from matings between Agrp-CRE Pomc-CRE Lepr-flox/flox (to be termed AGRP+POMC:Lepr−/− or occasionally A+P Lepr−/−) males and Lepr-flox/flox females. Due to occasional embryonic activity of the Agrp-CRE transgene, some Agrp-CRE-positive mice show deletion of both Lepr-flox alleles. These animals are readily detected by our genotyping protocol for the Lepr locus and were eliminated from subsequent analyses. The Lepr locus was genotyped with the following three primers to detect the flox (249 bp) and deleted/Δ17 (238 bp) Lepr alleles: mLepr-105, ACAGGCTTGAGAACATGAACAC; mLepr-106, GTCTGATTTGATAGATGGTCTT; and mLepr-65A, AGAATGAAAAAGTTGTTTTGG.
The Agrp-CRE and Pomc-CRE transgenes were detected was detected with the following primer sets with the expected product sizes (Agrp-CRE, ∼500 bp; Pomc-CRE, 90 bp): Agrp-CRE-F1, GTACCCTAAGGATGAGGAGAGAC; Agrp-CRE-R1, CCGCATAACCAGTGAAACAGCATTG; Pomc-CRE 3′-F, AACTAAGCGGCCGCCACCGC; Pomc-CRE, 3′-R GGCACTGGCTGCTCTCCAGG.
Mice were fed PicoLab Rodent Diet 20 (an irradiated mouse breeder chow with 20% protein and minimal 9% fat; LabDiet; PMI Nutrition International, St. Louis, MO) and water ad libitum unless stated. Genotyping was done on DNA obtained from ear clips. Blood was obtained by milking a nicked tail vein. All procedures were reviewed and approved by the institution’s animal care committee, conforming to accepted standards of humane animal care.
Energy balance studies: calorimetry, meal patterns, locomotion, and body temperature
Male mice A+P Lepr−/− 8 wk of age and AGRP-CRE 6 wk of age at the beginning of experiments served as subjects. Before data collection, animals were implanted with E-mitters (Mini-Mitter, Bend, OR) under ketamine/xylazine anesthesia for temperature measurements and allowed 4 d for recovery. Animals were individually housed in metabolic chambers maintained at 20–22 C on a 12-h light, 12-h dark cycle with lights on at 0700 h. Metabolic measurements (oxygen consumption, food intake, locomotor activity, core temperature) were obtained continuously using a CLAMS (Columbus Instruments, Columbus, OH) open circuit indirect calorimetry system. Mice were provided with 35% fat nutritionally complete powdered diet (Research Diets, New Brunswick, NJ) and tap water ad libitum. Presented results contain data collected over at least 7 d after at least 2–4 d of adaptation to the metabolic cages.
Under isoflurane anesthesia, mice received ip implants of radiofrequency impedance temperature probes (model G2; Minimitter). These probes were anchored to the interior face of the abdominal wall such that the intestines covered the probe. The abdominal incision was closed with 6–0 Prolene and the skin wound was closed with vetbond skin adhesive. After a 2-d recovery from surgery, animals were placed in chambers equipped with Minimitter ER-4000 receiver units that sampled core temperature from each mouse six times per minute continuously every day. Core temperatures were recorded directly onto a personal computer by Columbus Instruments temperature monitoring software interfaced with the Minimitter temperature receivers that registered core temperature.
To evaluate whether postweaning hyperphagia contributes to the obese phenotype of the adult AGRP+POMC:Lepr-flox mouse, feeding behavior was examined in a separate cohort of AGRP+POMC:Lepr-flox mice from 4 to 8 wk of age. Mice were maintained for 4 wk at 20–22 C on a 12-h light, 12-h dark cycle with lights on at 0700 h in individual chambers equipped with 20-mg pellet dispensers. Mice were provided with 35% fat nutritionally complete 20-mg pelleted diet (Research Diets) and tap water ad libitum. Food intake was measured continuously and body weight was assessed daily.
Leptin stimulation of arcuate nucleus signal transducer and activator of transcription (STAT)-3 phosphorylation
Mice were injected with leptin (10 mg/kg, ip) after an overnight fast. One hour after injection, the mice were anesthetized with ketamine/xylazine and perfused with 4% fresh paraformaldehyde. Brains were processed for phosphor-STAT3 immunostaining as described below.
Leptin-mediated inhibition of feeding
Ketamine/xylazine-anesthetized mice were implanted with third ventricle cannulae (coordinates: ML = 0, 2.5 mm posterior from bregma, 8.5 mm ventral to skull surface) and were housed individually on a 12-h light, 12-h dark cycle, with lights on at 0700 h. Cannula placement was functionally verified before leptin testing by 1 μl/1 μg peptide YY injection stimulating at least 1 g of food intake per 60 min in a nonfasted, daytime test. On each test day, testing began at 1800 h, 5 min after the intracerebroventricular injection of one of three doses (2.5, 5, 10 μg per 2 μl) of recombinant murine leptin (R&D Systems, Minneapolis, MN) or artificial cerebrospinal fluid vehicle (Harvard Apparatus, Holliston, MA). Food was presented in the form of 20 mg standard chow pellets, 3.8 kcal/g (Research Diets). Individual pellets were presented in a food trough, and timing of pellet removals was recorded. Pellet intake was calculated hourly for the first 6 h and then again at 23 h. All animals were tested with all doses, and data were analyzed with repeated-measures ANOVA with time and leptin dose and factors.
Hormone and glucose assays
Fasting serum insulin and leptin concentrations were done by ELISA (Alpco Diagnostics, Salem, NH). Glucose concentrations were determined by the glucose oxidase method (Glucometer; Bayer Corp., Tarrytown, NY).
Body mass and body composition determinations
Body mass was measured to the nearest 0.1 g. Body composition was determined by either dual-energy x-ray absorptiometry (Lunar Piximus; GE Health Care, Chalfont St. Giles, UK) or magnetic resonance spectroscopy using an ECHO magnetic resonance spectroscopy instrument (Echo Medical Systems, Houston, TX).
Microscopy and immunostaining
Mice were perfused with 4% formaldehyde through the left ventricle, and the fixed brains were equilibrated in 30% sucrose. For enhanced green fluorescent protein (GFP) and AGRP colocalization, coronal hypothalamic sections of 50 μm thickness were prepared with a vibratome. Sections were first blocked with 10% normal goat antiserum and then incubated in AGRP antiserum (1:500 dilution) or NPY antiserum (1:1500) with 0.4% Triton X-100 and 0.5% BSA in PBS for 48 h in a cold room, with gentle agitation. The primary antiserum was a rabbit polyclonal (Phoenix Pharmaceuticals, Inc., Burlingame, CA). After several washes with PBS, sections were incubated in biotinylated antirabbit IgG (1:50; Vector Laboratories, Burlingame, CA) for 2 h. After several washes in PBS, tissues were incubated in avidin conjugated with Texas Red (1:50; Vector Laboratories) for 3 h. Finally, tissues were washed for 30 min with PBS, mounted in Citifluor, and coverslipped. Native GFP and Texas Red fluorescence were visualized with the appropriate lasers and emission filters on a LSM 510 NLO multiphoton confocal microscope (Zeiss, Thornwood, NY).
For phosphorylated STAT (pSTAT)-3/AGRP colocalization, brains were cut into 30-μm coronal sections using a sliding microtome. Free-floating tissue sections were washed with PBS and pretreated with 1% H2O2 in methanol, 0.3% glycine in PBS, and 0.03% sodium dodecyl sulfate in PBS. Tissues were blocked in 3% normal donkey serum followed by incubation in rabbit anti-pSTAT3 (1:3000; Cell Signaling Technology, Danvers, MA) for 48 h at 4 C. Sections were then incubated with biotinylated donkey antirabbit (1:1000; Jackson ImmunoResearch, West Grove, PA) followed by Vectastain Elite ABC kit (Vector Laboratories) and 0.4% diaminobenzidine in 0.01% H2O2. Stained tissue slices were washed, blocked, and incubated with anti-AGRP (1:500; Phoenix Pharmaceuticals). Sections were labeled using goat antirabbit Alexa 594 (1:200; Molecular Probes, Invitrogen, Carlsbad, CA), mounted onto gelatin-coated slides, and coverslipped with ProLong Gold (Molecular Probes).
Images (×40 magnification) were captured using an FV-500 confocal microscope (Olympus, Center Valley, PA) and analyzed using Metamorph software with the operator blind to experimental group. Neurons exhibiting staining above threshold for both pSTAT3 and AGRP neurons were counted in 13 or more comparable 1-μm confocal slices per animal from three animals per group.
Data and statistical analysis
Data are expressed as means with sd or sem (specified in each figure or table). Comparisons between groups were performed using the unpaired one-tailed Student’s t test, one-way ANOVA repeated measures, or 2 × N ANOVA repeated measures using Prism (GraphPad Software, San Diego, CA). Post hoc analyses were conducted using Tukey post hoc or Bonferroni comparisons. Differences were significant with P ≤ 0.05.
To evaluate whether there was any positive or negative synergy (i.e. nonadditivity) between the effects of LEPR-B-null status in AGRP cells (denoted A) and LEPR-B-null status in POMC cells (denoted P), we regressed the dependent variables of interest (fat mass, lean mass, and fractional body fat) on A, P, and an A × P interaction term using ordinary least squares linear regression. We tested for the incremental effects of the interaction term at the nondirectional (i.e. two tailed) 0.05 α-level. The data indicated a pure additive effect of A × P on body composition.
Results
Specificity and efficacy of the Agrp-CRE transgene in eliminating LEPR-B expression
The Agrp-CRE transgene has been previously reported to be specific to AGRP cells, although specific neuronal counts have not been published (24). In Fig. 1 and Table 1 (as well as supplemental data S1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://endo.endojournals.org), we provide counts of the coincidence of native fluorescence from GFP with AGRP and NPY immunoreactivity in Agrp-CRE Z/EG mice, in which Z/EG is a reporter gene that expresses GFP under the control of a hybrid chicken β-actin promoter after the action of CRE recombinase. In fasted mice, we observed near complete concordance of the GFP signal with AGRP immunoreactivity. However, there is some variability between individual mice regarding the expression of CRE recombinase within AGRP neurons, ranging from 85 to 100%. We did not observe any GFP-positive neurons that were not also AGRP immunoreactive.
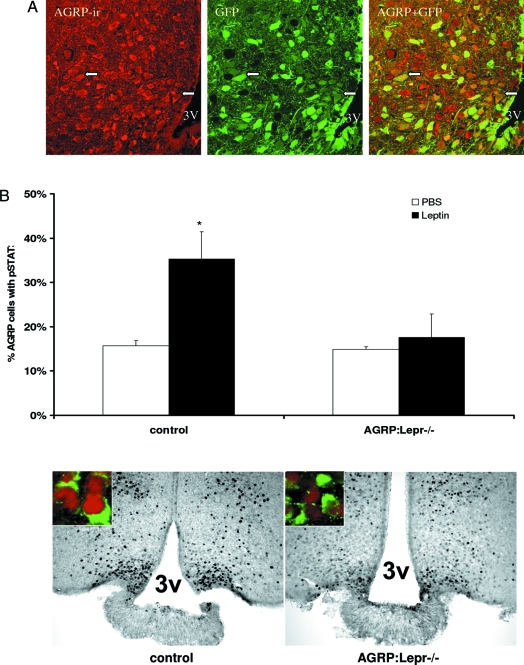
Figure 1.
Specificity of Agrp-CRE expression and efficacy of Lepr deletion. A, Native GFP fluorescence (GFP), activated by the Agrp-CRE transgene, colocalizes with AGRP immunoreactivity (AGRP-ir and visualized with a Texas Red conjugate) in images obtained by confocal microscopy of the arcuate nucleus in coronal brain sections. The AGRP staining shows some neurons with a smooth intense cytoplasmic stain, whereas some cells show punctate staining suggestive of vesicularly delimited immunoreactive material. White arrows point to some of the cells labeled with both AGRP-ir and GFP fluorescence. The merged images show near complete overlap of the two signals, after allowing for the large range of signal intensities in both channels. Some predominantly green neurons in the merged image (due to an intense green signal for the GFP channel) are AGRP-ir positive albeit weakly positive, whereas there are some AGRP-ir-positive neurons that are clearly GFP negative. 3V, Third ventricle. B, Quantification of STAT3 phosphorylation after leptin treatment in Agrp-CRE Lepr-flox/flox mice and control mice. Mice were fasted overnight, injected with leptin (10 mg/kg, ip), and perfused 1 h after injection. The bars represent the means and sds for three mice in each group. *, Significant difference (P < 0.05). The lower panel shows representative images of the dual visualization of AGRP immunoreactivity and pSTAT3 for control mice and Agrp-CRE Lepr-flox/flox (AGRP:Lepr−/−) 1 h after leptin injection (10 mg/kg, ip). The pSTAT3 gray-scale image was obtained from diaminobenzidine-developed sections. For the two color images, the gray-scale image was processed to mimic red fluorescence (darkest pixels showing most intense red pixels) and registered with the green channel for the AGRP immunostain. Note that the control mice reveal many more pSTAT3-labeled nuclei within the medial arcuate nucleus.
Table 1.
Colocalization of AGRP and NPY immunoreactivity with GFP native fluorescence due to Agrp-CRE transgene mediated activation
| Mouse ID | AGRP+ GFP+ | AGRP+ only | GFP+ only |
|---|---|---|---|
| 435 | 84 | 0 | 0 |
| 436 | 85 | 10 | 0 |
| 437 | 56 | 0 | 0 |
| 445 | 50 | 0 | 0 |
| Total | 275 (96.5%) | 10 (3.5%) | 0 |
| Mouse ID | NPY+ GFP+ | NPY+ only | GFP+ only |
| 436 | 43 | 7 | 0 |
| 437 | 50 | 0 | 0 |
| Total | 93 (93%) | 7 (7%) | 0 |
Agrp-CRE Lepr-flox/floxanimals were fasted overnight prior to perfusion with paraformaldehyde to fix brain tissues. Thick (50–200 μm) sections were cut without freezing to preserve native GFP fluorescence and stained for AGRP and NPY immunoreactivity (Texas red conjugated secondary antibody). Images were obtained by confocal microscopy with 2–5 μm optical sections using a ×20 objective. Counts of GFP-positive neurons and AGRP- and NPY-ir neurons were performed on color-separated images. Neurons are classified below and neuron counts are tabulated for individual mice.
To assess the functional removal of LEPR-B from AGRP neurons in Agrp-CRE Lepr-flox/flox animals, we examined the presence of immunologically detectable phosphorylation of STAT3 in AGRP-immunoreactive (ir) neurons in leptin-treated animals. Activation of LEPR-B rapidly and robustly mediates the phosphorylation of STAT3 in the brain, and the immunohistochemical detection of pSTAT3 provides a sensitive assay for leptin action in individual leptin-responsive neurons. In control mice (Lepr-flox/flox no CRE), there is a doubling of the number of AGRP neurons that are pSTAT3 positive. In Agrp-CRE Lepr-flox/flox mice, leptin treatment did not increase the number of AGRP neurons that are pSTAT3 positive (Fig. 1B). Thus, the majority of AGRP neurons in Agrp-CRE Lepr-flox/flox, hereafter called AGRP:Lepr−/−, mice are devoid of LEPR-B action.
Increased body mass and fat mass due to selective Lepr deletion
We generated mice with Lepr deletion in AGRP/NPY and POMC/CART neurons by intercrossing mice of the following genotypes: Agrp-CRE Lepr-flox/flox [AGRP:Lepr−/−] mated to Pomc-CRE Lepr-flox/flox [POMC:Lepr−/−]. Subsequently we used Agrp-CRE Pomc-CRE Lepr-flox/flox males [AGRP+POMC:Lepr−/−] mated to Lepr-flox/flox females for all subsequent experiments because this type of mating would produce four types of mice in equal proportions in all litters: AGRP+POMC:Lepr−/−; AGRP:Lepr−/−; POMC:Lepr−/−; and Lepr-flox/flox. We used Lepr-flox/flox females to avoid any potential effects that maternal obesity, as would be the case if we used either POMC:Lepr−/− or AGRP:Lepr−/− mice as dams, might have on progeny development. Finally, all mice were typed for Lepr in ear or tail tissues to identify and eliminate those mice with complete or nearly complete deletion of both alleles due to early embryonic activation of the Agrp-CRE transgene with approximately 25% of all Agrp-CRE-positive mice being eliminated from the studies (25).
We studied several cohorts of male and female mice at 2–3 months of age and observed significant body weight differences due to genotype (Table 2). These studies were mainly conducted with one Agrp-CRE transgenic line (25) and confirmed with a second Agrp-CRE line (23) in an independent cohort. AGRP:Lepr−/− mice and POMC:Lepr−/− mice were equivalent in weight and heavier than the control group. In addition, these cohorts were raised at three different institutions’ vivariums, indicating that the phenotypes are robust and reproducible. The AGRP+POMC:Lepr−/− groups showed the largest masses among all of the groups.
Table 2.
Effect of loss of Lepr in AGRP and/or POMC neurons on body mass and body composition
| AGRP+POMC:Lepr−/−(a) | AGRP:Lepr−/−(b) | POMC:Lepr−/−(b) | No CRE (c) | |
|---|---|---|---|---|
| Body mass | ||||
| Males, A | 41.1 ± 5.6 | 34.3 ± 4.7 | 35.9 ± 6.7 | 31.7 ± 3.5 |
| Males, B | 37.3 ± 2.9 | 35.0 ± 6.7 | 34.2 ± 2.2 | 29.5 ± 1.7 |
| Males, C | 34.3 ± 6.5 | 32.4 ± 4.7 | 31.4 ± 3.9 | 27.1 ± 3.2 |
| Females, A | 40.8 ± 7.3 | 32.6 ± 4.1 | 30.5 ± 3.7 | 25.9 ± 3.1 |
| Females, B | 44.5 ± 3.3 | 37.5 ± 5.4 | 35.4 ± 4.3 | 30.8 ± 3.6 |
| Females, C | 35.3 ± 3.5 | 30.1 ± 4.3 | 28.9 ± 0.5 | 23.3 ± 2.1 |
| Females, D | 31.1 ± 3.3 | 29.0 ± 3.1 | 29.9 ± 2.7 | 25.2 ± 3.2 |
| Fractional fat composition | ||||
| Males, B | 0.336 ± 0.030 | 0.268 ± 0.047 | 0.277 ± 0.47 | 0.223 ± 0.049 |
| Females, A | 0.438 ± 0.039 | 0.382 ± 0.049 | 0.362 ± 0.047 | 0.282 ± 0.019 |
| Females, B | 0.497 ± 0.060 | 0.441 ± 0.120 | 0.331 ± 0.112 | 0.271 ± 0.048 |
| Females, C | 0.388 ± 0.067 | 0.353 ± 0.097 | 0.283 ± 0.063 | 0.210 ± 0.067 |
| Females, D | 0.368 ± 0.094 | 0.307 ± 0.077 | 0.293 ± 0.058 | 0.158 ± 0.037 |
Body mass of male and female mice (2–3 months of age) of four genotypes are provided. Three cohorts of each sex were raised and analyzed over a 24-month period at two different institutions. Weights in grams are provided as means and sd for each genotype group, and group sizes ranged from four to 12 in each cohort, designated A, B, or C. Fractional fat composition by dual-energy x-ray absorptiometry was measured in selected cohorts. Letters under each genotype denote a significant difference, compared with other genotypes (pairwise analyses done with Wilcoxon matched-pair signed rank test, P < 0.05). Genotypes showing no significant differences share the same letter designation. An analysis for significant differences between all groups for body mass and body composition was done with the Friedman test (P < 0.001).
Body compositional analysis shows that the increased fat mass is accounted by fat accumulation (Table 2) because there was no increase in lean body mass in the AGRP:Lepr−/− (23.1 ± 2.7 g), POMC:Lepr−/− (23.8 ± 3.5 g), and AGRP+POMC:Lepr−/− (25.3 ± 3.9 g) groups, compared with control non-CRE mice (22.4 ± 1.4 g, male data presented). As was the case for body mass, body fat fraction was equivalent between AGRP:Lepr−/− and POMC:Lepr−/− mice, which was increased over the control groups, whereas the AGRP+POMC:Lepr−/− groups had the highest fractional body fat contents.
We addressed the issue of additive or synergistic effect on body mass in the AGRP+POMC:Lepr−/− mice. Because POMC neurons receive projections with inhibitory neurotransmitters from AGRP/NPY neurons, it is possible that we might observe synergistic effects due to loss of LEPR in both neuronal groups. Regression analysis was performed for fat mass, lean mass, and fraction body fat content to detect positive or negative synergy due to selective Lepr deletions. There was no compelling evidence for an interaction factor for any of the three dependent variables (all P > 0.5). These results indicate that a simple additive model for body mass and body fat mass is sufficient to explain the effects of loss of leptin signaling in AGRP/NPY and POMC neurons. A similar result of additive effects has been observed for mice with loss of leptin receptors in both POMC and steroidogenic factor (SF)-1 neurons (8).
Mild hyperinsulinemia due to selective LEPR ablation
One of the hallmarks of leptin signaling deficiency is insulin resistance, hyperglycemia, and hyperinsulinemia. Fasting blood glucose concentrations were statistically equivalent across all four groups (data not shown). However, we observed mild fasting hyperinsulinemia in male and female AGRP+POMC:Lepr−/− mice (Fig. 2A). We did not observe hyperinsulinemia in either the AGRP:Lepr−/− or POMC:Lepr−/− groups. Testing of the AGRP+POMC:Lepr−/− mice with a glucose tolerance test did not reveal glucose intolerance (Fig. 2B) with the areas under the curve being equivalent (control mice: 17,315 ± 2,400 vs. AGRP+POMC:Lepr−/− mice: 16,172 ± 1,586; units in milligrams per deciliter per minute) to control mice.
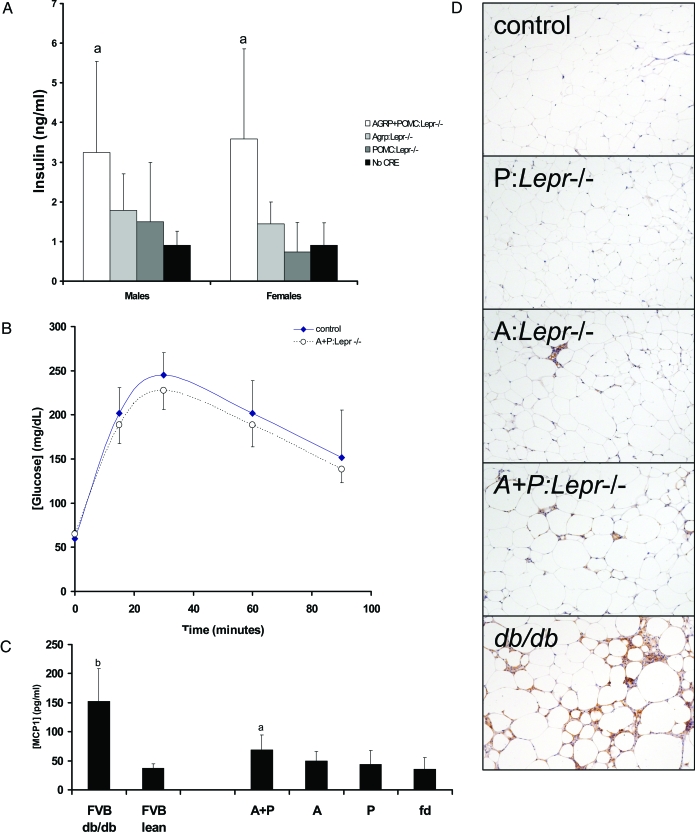
Figure 2.
Glycemic control and adipose morphology with cell type-specific ablation of Lepr. A, Fasting insulin concentrations show an increase in the AGRP+POMC:Lepr−/− mice only. Neither the AGRP:Lepr−/− nor the POMC:Lepr−/− mice show increased insulin concentrations over the No CRE group (group sizes were between six and eight for each group with equal numbers of males and females in each group; analysis (not shown showed no sexual dimorphism). B, Glucose tolerance testing of AGRP+POMC:Lepr−/− mice. Glucose was given ip (0.75 mg/g), and glucose concentrations were measured from tail blood. No difference was observed between the two genotypes (n = 6 for each group). C, Circulating concentrations of MCP1 (chemokine ligand 2). The bars represent means and sds for six groups of mice: FVB db/db; FVB lean; AGRP+POMC:Lepr−/− (A+P); AGRP:Lepr−/− (A); POMC:Lepr−/−; No CRE (fd). Groups that are significantly different from the control groups [FVB lean and No CRE(fd)] are designated with a letter above the bar (n = 6 for each group). D, Photomicrographs of adipose tissue sections stained for a macrophage-specific membrane marker (F4–80).
Increased adiposity is associated with increased numbers of macrophages within the adipose tissue. We observed increased numbers of cells that were staining positively for the macrophage cell surface marker F4–80 within the adipose tissue of mice with selective Lepr deletions (Fig. 2D), although the degree of macrophage infiltration was not equivalent to that observed in FVB db/db mice. We determined the circulating concentrations in female mice of one of the chemokines associated with macrophage infiltration of adipose tissue, monocyte chemotactic protein (MCP)-1 or chemokine ligand-2. The AGRP+POMC:Lepr−/− female mice had a significant increase of MCP1 over control mice (Fig. 2C). Whereas the AGRP:Lepr−/− and POMC:Lepr−/− group had apparent increases of circulating MCP1, they did not reach statistical significance.
Fertility and lactation capacity are preserved in AGRP+POMC:Lepr−/− mice
All of the mice were produced by using AGRP+POMC:Lepr−/− males as studs. We formally documented their reproductive capacity in test matings (Table 3). Of four males tested with virgin females, all four males produced progeny within 21–39 d of mating. Female AGRP+POMC:Lepr−/− mice are also fertile, delivering their first litters within 21 d of the initial pairing date. Because the average gestation time in mice is 20 d and the average estrus cycle is 4 d, these matings produced successful pregnancies within one estrous cycle. The numbers of pups born to and weaned by the female A+P:Lepr−/− mice are similar to Lepr-flox/flox mice, indicating that lactational capacity was not compromised.
Table 3.
Fertility of AGRP+POMC:Lepr−/− mice
| AGRP+POMC:Lepr−/− | No. tested | No. of fertile mice | Days to delivery | Litter size | Pups weaned |
|---|---|---|---|---|---|
| Males | 4 | 4 | 27 ± 8.5 | nd | nd |
| Females | 3 | 3 | 20.7 ± 0.6 | 7.7 ± 0.6 | 6.7 ± 1.5 |
Virgin AGRP+POMC:Lepr−/− males at 10–12 wk of age were mated with virgin 8-wk-old females. Virgin AGRP+POMC:Lepr−/− females at 8–10 wk of age were mated with known fertile males. Pregnancies and dates of delivery were recorded for each male and female. In addition, the numbers of pups born and weaned are recorded for female A+P:Lepr−/− females. Numbers represent the averages and sd of each variable.
nd, Not determined.
Hyperphagia and lower energy expenditure of periweaning AGRP+POMC:Lepr−/− mice is suppressed in adults
We observed that AGRP+POMC:Lepr−/− mice gain weight more rapidly after weaning (Fig. 3). Consequently, we examined meal feeding patterns of periweaning age (between 4 and 7 wk) and adult age (12–16 wk) of AGRP+POMC:Lepr−/− males and their age-matched control siblings. Periweaning AGRP+POMC:Lepr−/− mice displayed a small but significant difference in body weight at 4 wk of age yet with almost double the fat mass (Fig. 3A). These mice also gain weight more rapidly than controls, and this was entirely due to an increase in adiposity. Periweaning AGRP+POMC:Lepr−/− mice were significantly hyperphagic throughout wk 4–8 (Fig. 3B). This hyperphagia was due to increased meal size rather than an increase in meal number. Because fat mass was accruing during this period of rapid weight gain, we measured leptin concentrations over this period because an increase in circulating leptin concentrations could lead to activation of compensatory responses in the remaining leptin-responsive neurons. Surprisingly, leptin concentrations were highly elevated in AGRP+POMC:Lepr−/− mice at 4 wk of age with no significant increase over time (Fig. 3C). This suggests that alterations in the metabolic and ingestive phenotypes between periweaning and adult AGRP+POMC:Lepr−/− mice is not due to increases of leptin concentrations.
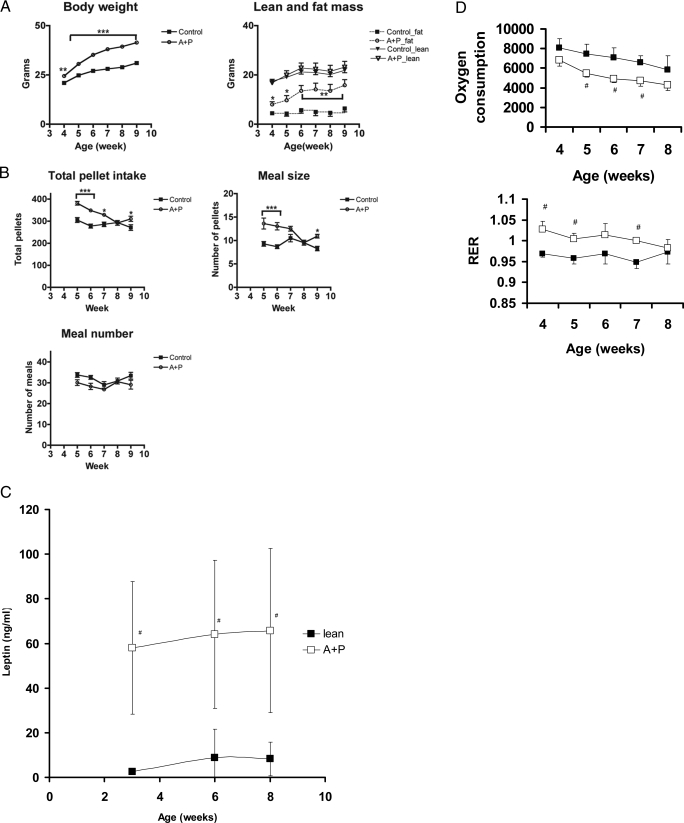
Figure 3.
Periweaning hyperphagia and oxidative sparing of triglycerides in AGRP:POMC:Lepr−/− mice. A, Body mass of male AGRP+POMC:Lepr−/− mice diverges quickly from control male mice immediately after weaning and rate of weight gain appears to parallel the weight gain of control mice by 9 wk of age. The increase in body mass is due to an increase in body fat, whereas lean mass was not increased. Data shown are means and sds; there were eight mice in each group for A–D. B, An analysis of meal size and meal number of postweaning AGRP+POMC:Lepr−/− male mice indicated hyperphagia is most prominent from wk 5 to wk 7. Total pellet intake was greater for the AGRP+POMC:Lepr−/− mice. Intakes during both the light and dark phases of the day are increased. C, Leptin concentrations during the postweaning period. Note the elevated leptin concentrations of AGRP+POMC:Lepr−/− mice over controls over the entire postweaning period (#, Difference of the means with P < 0.05). D, Indirect calorimetry indicates that the lower energy expenditure of young AGRP+POMC:Lepr−/− mice (open symbol) compared with control mice (filled symbol) is also associated with higher RER. #, Difference due to genotype for that time period.
Indirect calorimetry was used to measure oxygen consumption in periweaning AGRP+POMC:Lepr−/− male mice (Fig. 3D). The mice with selective Lepr deletion had approximately 12% decreased oxygen consumption consistently during the 4 wk of measurement, relative to control siblings. The calorimetry data also showed a slight but significant increase in the respiratory exchange ratio (RER) of the AGRP+POMC:Lepr−/− mice, indicative of oxidative sparing of triglycerides. Whereas the magnitude of the difference is apparently small, about 0.05, this represents about 16.7% of the range for RER values under typical circumstances (see below for a test of the significance of RER alterations with a high fat feeding regimen; Fig. 4E). Calculations of energy expenditure without taking into account alterations in body composition for these mice (both wild type and mutant) are likely to be misleading because they are in a phase of rapid weight gain.
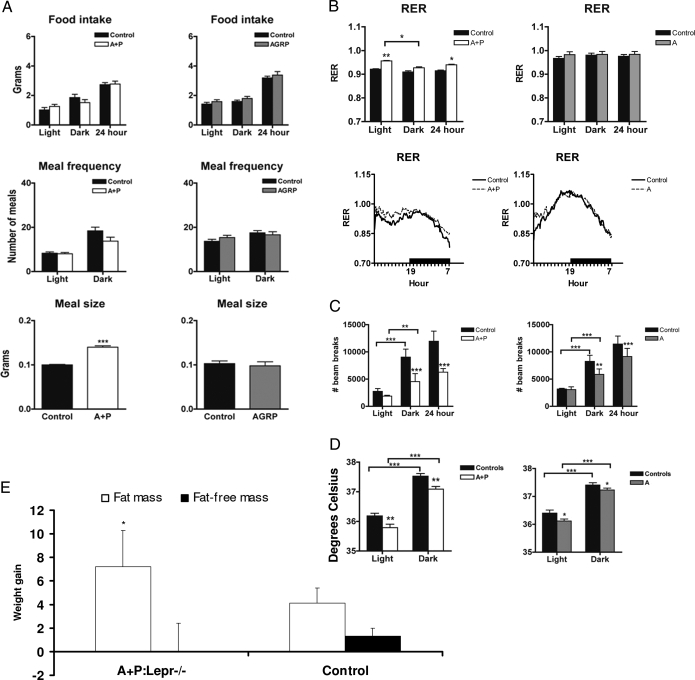
Figure 4.
Analysis of adult ingestive behavior and metabolism after cell-type ablation of LEPR. A, Food intake, meal frequency, and meal size were determined for adult (>3 months old) AGRP+POMC:Lepr−/− (A+P) and AGRP:Lepr−/− (AGRP) male mice and their respective controls. The bars represent means and sds (A–E). Food intake and meal frequency did not differ between the groups over the light and dark phases. Meal size was increased in AGRP+POMC:Lepr−/− (A+P) mice, signified with asterisks. There were eight mice in each group (A–E). B, Monitoring of RER over a 7-d period shows an increased RER for AGRP+POMC:Lepr−/− mice (denoted by asterisks) but no alterations in AGRP:Lepr−/− mice. This increased RER is observed only during the light phase of the day (subjective dark). The data are presented over a 24-h period as well as presented as bars for the light and dark phases. C, Activity, as defined as beam breaks caused by locomotion, is significantly reduced during the dark phase of the day for both AGRP+POMC:Lepr−/− and AGRP:Lepr−/− male mice. Data are presented as means and sds. D, Body temperatures for both AGRP+POMC:Lepr−/− and AGRP:Lepr−/− male mice are significantly reduced (denoted with asterisks) during the light and dark phases. E, Gains in fat mass and fat-free mass of AGRP+POMC:Lepr−/− mice during feeding of a 60% fat diet. The AGRP+POMC:Lepr−/− mice gain significant amounts of fat mass (*, P < 0.05), compared with the control group. There is no significant difference in the gains of fat-free mass between the two groups.
We also examined food intake and meal ingestion patterns in adult (3–4 months) male AGRP+POMC:Lepr−/− mice as well as male AGRP:Lepr−/− mice (Fig. 4A). Measures of daily food intake and energy expenditure among AGRP+POMC:Lepr−/−, AGRP:Lepr−/−, and control mice at 3–4 months of age did not demonstrate any differences between the groups (Table 4), despite the demonstrable differences in body mass and body composition. Examination of meal-feeding patterns showed that AGRP+POMC:Lepr−/− mice have increased meal size relative to control mice (Fig. 4A). AGRP:Lepr−/− mice did not show any alterations in total daily intake, meal size, or meal size.
Table 4.
Energy balance in A+P:Lepr−/− and A:Lepr−/− mice
| Caloric intake (kcal/d) | Caloric expenditure (kcal/d) | Body mass (g) | |
|---|---|---|---|
| A+P:Lepr−/− | 12.22 ± 0.88 | 11.39 ± 0.63 | 42.22 ± 0.65a |
| No CRE | 11.99 ± 0.86 | 11.39 ± 0.21 | 35.18 ± 0.60 |
| A:Lepr−/− | 14.88 ± 0.52 | 13.40 ± 0.22 | 35.21 ± 1.59b |
| No CRE | 14.05 ± 1.05 | 13.23 ± 0.21 | 28.52 ± 0.82 |
Energy expenditure was measured by indirect calorimetry and based on calories expended per mouse. Caloric intake is based on cumulative chow intakes. Data are presented as means and sem. No statistically significant difference was observed in the two cohorts (n = 8 for each cohort) for caloric intake or caloric expenditure. There was a difference in body mass (P < 0.05).
Significant difference between A+P:Lepr−/− mice and control mice.
Significant difference between A:Lepr−/− and control mice.
Oxidative sparing of fat in AGRP+POMC:Lepr−/− mice leads to increased fractional body fat
Examination of the energy intake and energy expenditure of adult 3- to 4-month-old AGRP+POMC:Lepr−/− mice showed no differences in total caloric intake and expenditure by indirect calorimetry relative to control sibling mice, suggesting that both types of animals were in neutral energy balance (Table 4). To provide a mechanism for the increased body fat fraction of AGRP+POMC:Lepr−/− mice, we examined the RER (or Rq) as a measure of the composition of oxidized nutrients. For AGRP+POMC:Lepr−/− adult males, we saw a significant increase in the RER, relative to control mice (Fig. 4B), as was observed in periweaning AGRP+POMC:Lepr−/− mice. We did not observe a change in RER for AGRP:Lepr−/− mice (Fig. 4B).
Despite the data regarding neutral energy balance for adult mice of all genotypes, we observed a significant effect of selective leptin receptor deletion on physical activity and core body temperature. AGRP:Lepr−/− mice and AGRP+POMC:Lepr−/− mice showed decrements in physical activity in the dark phase of the circadian cycle (Fig. 4C). There was also a significant decrease of core body temperature for both types of mice during the entire circadian cycle (Fig. 4D).
The altered RER of AGRP+POMC:Lepr−/− mice would be expected to make them accumulate more fat during feeding of a high-fat diet because more of the ingested calories would be deposited within adipose tissue than in control mice. We placed adult female mice of two genotypes (A+P:Lepr−/− and non-CRE) on a 60% fat diet. Total weight accumulation and fat mass accretion was significantly higher in A+P:Lepr−/− mice after 5 wk of high-fat feeding (Fig. 4E), although total food intake was not different between the groups (data not shown). There was no significant difference in accretion of fat-free mass during the interval of high-fat feeding in the two groups of mice.
Reduced inhibition of feeding from central leptin in AGRP+POMC:Lepr−/− mice
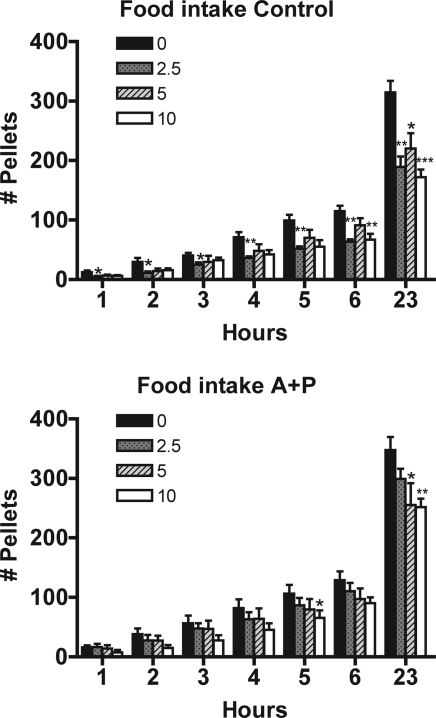
To address the issue of whether leptin sensitivity was functionally altered after the loss of LEPR-B expression in AGRP/NPY and POMC neurons, we tested the ability of central leptin injections to inhibit feeding. Two groups of adult mice, AGRP+POMC:Lepr−/− males and non-CRE males, were injected with varying doses of leptin (2.5, 5, and 10 μg) through a cannula situated in the third ventricle. In control mice, leptin at all doses caused a decrease in the 23 h cumulative intake (Fig. 5). There was also a decrease at the 6-h cumulative intake for the 2.5- and 10-μg doses. In the AGRP+POMC:Lepr−/− mice, there was a significant decrease in the 23-h cumulative intake for the 5- and 10-μg doses, whereas 2.5 μg did not produce feeding inhibition that reached statistical significance. Moreover, leptin did not produce any statistically significant decreases in feeding except at 5 h for the 10-μg dose.
Figure 5.
Inhibition of feeding by central leptin in AGRP+POMC:Lepr−/− mice. The cumulative food intake of male mice (control and AGRP+POMC:Lepr−/−) fitted with lateral ventricular cannulae was monitored for up to 23 h. Doses of leptin (2.5, 5, and 10 μg or vehicle) were injected immediately before monitoring intake. *, Statistically significant difference from the vehicle treatment. There were six mice in each group and the data are presented as means and sds.
Discussion
Postweaning hyperphagia and oxidative sparing of fats due to loss of leptin receptors in AGRP/NPY and POMC neurons
Our data indicate that leptin exerts a coordinated modulation of the energy homeostatic functions of hypothalamic POMC and AGRP/NPY neurons. Some of the effects of leptin modulation appear to be additive, such as control of body mass and fractional fat mass. Some effects of leptin modulation of these two types of neurons appear to be nonlinear or nonadditive, such as meal feeding patterns and RER. Furthermore, hyperphagia is only apparent in young periweaning animals with loss of leptin signaling in both POMC and AGRP/NPY neurons.
Transient hyperphagia in the AGRP+POMC:Lepr−/− mice is surprising because complete leptin signaling deficiency leads to persistent hyperphagia in adolescent and adult rodents, a behavioral trait that is found to contribute significantly to the degree of obesity attained (26,27). Postweaning weight gain is a trait that is a highly heritable phenotype (28,29,30) and is associated with alterations in body composition that can confound balance studies. Our current data suggest that the function of hypothalamic POMC and AGRP/NPY neurons may be a significant factor during postweaning weight gain in some models. The mechanism for the decrease of AGRP+POMC:Lepr−/−animals’ food intake to control levels over time is clearly unrelated to circulating leptin concentrations. The attenuation of the ability of leptin to inhibit feeding in adult AGRP+POMC:Lepr−/− mice provides evidence that the mechanism for normalization of feeding in adult AGRP+POMC:Lepr−/− mice is leptin independent.
The hyperphagia in developing AGRP+POMC:Lepr−/− mice is manifested by a specific increase in meal size with no change in meal frequency. Leptin’s effect on feeding occurs through reducing meal size without affecting meal number (31,32). Moreover, leptin modulates other signals related to feeding and satiety, e.g. the gut hormone cholecystokinin (CCK) (12). Obese Leprfa(k)/fa(k) rats have a markedly increased meal size and reduced satiety in response to CCK. Because these animals are leptin receptor deficient, this observation suggests a critical role for leptin in the response to endogenous signals that induce satiety. The arcuate nucleus appears to be critical because when adenoviral gene therapy was used to express either functional leptin receptors or a reporter gene in this area of Leprfa(k)/fa(k) rats, the effect of CCK on the activation of neurons in the nucleus of the solitary tract and area postrema were normalized (12). We note that CCK’s satiety effect is ineffective in younger rats but attains potency in adult rats (33).
A simple model to be derived from this study is that leptin, at all ages, inhibits feeding via combined actions on AGRP/NPY and POMC neurons. The loss of leptin regulation of these neurons results in hyperphagia. Maturation of the controls of feeding is manifested by the additional overlay of other, leptin-independent mechanisms of satiety and modulators of meal size that are likely to include CCK. The presence or absence of these leptin-independent mechanisms are likely to be responsible for the phenotypes of mice with loss of AGRP neurons. Adults with these satiety mechanisms are unable to overcome the feeding inhibitory influences, whereas perinatal mice, which have not developed these mechanisms, remain unaffected. We assume that some degree of plasticity permits compensatory changes for the perinatal loss of AGRP neurons with normal feeding patterns.
Maintenance of obesity without hyperphagia in adult A+P:Lepr−/− mice
There is an apparent increased metabolic efficiency in AGRP+POMC:Lepr−/− mice in that their increased mass is maintained by a daily food intake that is isocaloric to control mice that have a lower body mass. However, because the increased body mass is primarily in triglyceride mass, which does not impose a large metabolic burden, the normophagia of adult AGRP+POMC:Lepr−/− animals is sufficient to maintain their increased body mass. We should note that the increased RER of adult AGRP+POMC:Lepr−/− mice permitted increases in body fat content greater than that observed in control mice. Thus, oxidative sparing of triglycerides can contribute to increased body fat content without hyperphagia, at least with high dietary fat.
The AGRP+POMC:Lepr−/− mice have decreased core temperature and locomotor activity. This must have some impact on energy expenditure, although it is not possible to calculate the direct caloric equivalents. Because total daily energy expenditure was not altered, some other component of energy output must be increased in A+P:Lepr−/− mice. Whereas diet-induced thermogenesis is a likely candidate as the balancing mechanism, it would be difficult to calculate the energetic equivalents among diet-induced thermogenesis, body temperature maintenance, and locomotor activity in mice.
Synergism between POMC and AGRP/NPY arcuate neurons
Studies of rodents with genetic manipulations of both POMC and AGRP/NPY neurons have been previously reported (12,17,18). However, these were performed by ablations of the neurons. These manipulations would be expected to antagonize each other because the loss of POMC neurons leads to hyperphagia and obesity, whereas the loss of AGRP/NPY neurons causes hypophagia and weight loss in adults. Surprisingly, the loss of AGRP/NPY arcuate neurons in neonates does not affect feeding or subsequent growth, setting a precedent for developmental influences on AGRP/NPY neuronal function. In contrast, our manipulation of LEPR expression would have been expected to enhance obesity and insulin resistance.
We observed the appearance of elevated circulating insulin, leptin, and MCP1 concentrations in AGRP+POMC:Lepr−/− mice, whereas AGRP:Lepr−/− mice did not show such a similar overall pattern. Leptin concentrations were increased in AGRP:Lepr−/− mice, but insulin concentrations were significantly increased over control mice. We were surprised that we did not observe more severe perturbations in glucose control because widespread but incomplete loss of LEPR in the brain leads to hyperglycemia and insulin resistance (34,35). It is possible that the hyperleptinemia of mice with partial Lepr deficiency has a beneficial compensatory effect by activating the remaining LEPR-B-bearing neurons, minimizing the impact of the genetic ablation of Lepr within the arcuate nucleus.
Models of LEPR-B replacement into the arcuate nucleus of LEPR-deficient animals have been studied and reported (34,35). Food intake and weight gain were reduced in male LEPR-deficient rats with viral LEPR-B replacement in the arcuate nucleus. Unfortunately, long-term changes measured in months could not be assessed due to the transient expression provided by the adenoviral vector. Estrous cycling and hormonal concentrations were normalized in female LEPR-deficient rats with arcuate nucleus delivery of LEPR-B. These studies provide a complementary view to the role of LEPR-B-expressing arcuate neurons. Two explanations, which are not mutually exclusive, can be proposed to reconcile these two reports with our current studies: 1) non-POMC, non-AGRP/NPY arcuate nucleus neurons that express LEPR-B contribute to the control of feeding and the hypothalamic-pituitary-gonadal axis; and 2) extra-arcuate nucleus LEPR-B-bearing neurons contribute in a significant manner to leptin’s actions of feeding and reproduction. The identity of non-POMC, non-AGRP/NPY arcuate nucleus LEPR-B neurons remains to be determined, whereas there are many known extra-arcuate nucleus sites that bear leptin-responsive neurons.
A mouse model of leptin receptor reactivation in the arcuate nucleus in otherwise LEPR-B signaling-deficient animals found that LEPR reactivation reduced body weight and food intake, improved glucose homeostasis, and normalized locomotor activity, whereas male fertility was not restored (20). Our findings of reduced locomotion and normal fertility in deletion of LEPR signaling in POMC and AGRP/NPY arcuate neurons is consistent with this report. The findings on food intake and body weight regulation are also not inconsistent between the two studies. However, a major difference is the difference in glucose homeostasis between the models of LEPR reactivation in adult mice and LEPR deletion during embryogenesis and periweaning. Because complete LEPR deficiency by either germline mutations or CRE-mediated excision in the FVB strain of the current study causes severe hyperglycemia (34,36), it is unlikely that the difference is due to strain differences between the two studies. We cannot exclude the possibility of developmental alterations that may have occurred in the AGRP+POMC:Lepr−/− mice. Other possibilities are the existence of another cell type within the arcuate nucleus that is critical to glucose homeostasis or extra-arcuate nucleus neurons that are directly leptin responsive contribute significantly to glucose control when arcuate nucleus neurons are rendered LEPR deficient.
We should note that the AGRP:Lepr−/− and the AGRP+POMC:Lepr−/− mice have reduced locomotor activity. Reactivation of LEPR in the arcuate nucleus restored locomotor activity to normal (20). This is highly suggestive that the AGRP/NPY neurons are responsible for mediating leptin’s control of locomotor activity.
The role of extraarcuate sites of leptin responsivity
A surprising finding of our studies is the incomplete replication of the full phenotype of LEPR deficiency. The A+P:Lepr−/− animals are fertile, normoglycemic with very mild hyperinsulinemia, and normophagic as adults. These observations would be highly suggestive that there must be other extraarcuate neurons that mediate leptin’s actions. There are known LEPR-B-expressing neurons within the paraventricular nucleus, the lateral hypothalamic area, the ventromedial nucleus, and the dorsomedial nucleus (37). Because all of these sites can alter body mass when bilateral lesions are performed and show STAT3 phosphorylation on leptin stimulation, it is likely that some, if not each, of these nonarcuate sites must be mediators of leptin function. The ventromedial nucleus neurons that express SF1 have been critically evaluated (8) and are important in leptin’s control of body mass and body composition. Moreover, mice with loss of LEPR in POMC and SF1 neurons showed additive effects on body weight. This point of view is supported by our report that two different transgenes driving LEPR-B expression with varying expression in hypothalamic nuclei from different neuronal-specific promoters were necessary to normalize body mass, body composition, fertility, and glycemic control in db/db mice (37). Interactions between these sites probably contribute to the regulation of meal feeding because loss of leptin modulation of POMC and AGRP/NPY neurons could produce only transient hyperphagia. However, leptin’s modulation of puberty/fertility and glycemic control appear to require one or more hypothalamic sites that have not yet been identified.
In summary, all of the phenotypic manifestations of complete loss of leptin signaling, except for reproductive capacity, are displayed in mice with loss of leptin receptors in POMC and AGRP neurons, albeit to a lesser degree and in a developmentally controlled manner. Therefore, we conclude that the combined output from POMC and AGRP/NPY neurons is a major efferent mediator of leptin action.
Supplementary Material
Footnotes
This work was supported by Grants DK057621 (to S.C.C.), DK026687 (to G.J.S. and S.C.C.), DK020541 (to G.J.S.), and DK063608 (to S.C.C.), DK066618 (to G.J.S.) and Skirball Foundation (to G.J.S. and S.C.C.), DK056116 (to B.B.L.) and DK053301 (to J.E.), DK056336 (to D.B.A.), DK056731 (to M.G.M.), and 5P30DK0462 (to B.B.L.).
Disclosure Statement: The authors have nothing to declare.
First Published Online December 27, 2007
Abbreviations: AGRP, Agouti gene-related peptide; CART, cocaine amphetamine regulated transcript; CCK, cholecystokinin; CRE, cyclization recombinase; GFP, green fluorescent protein; ir, immunoreactive; LEPR, leptin receptor; Lepr, leptin receptor gene; MCP, monocyte chemotactic protein; NPY, neuropeptide Y; POMC, proopiomelanocortin; pSTAT, phosphorylated STAT; RER, respiratory exchange ratio; SF, steroidogenic factor; STAT, signal transducer and activator of transcription.
References
- Leibel RL, Chung WK, Chua Jr SC 1997 The molecular genetics of rodent single gene obesities. J Biol Chem 272:31937–31940 [DOI] [PubMed] [Google Scholar]
- Elmquist JK 2001 Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav 74:703–708 [DOI] [PubMed] [Google Scholar]
- Alingh Prins A, de Jong-Nagelsmit A, Keijser J, Strubbe JH 1986 Daily rhythms of feeding in the genetically obese and lean Zucker rats. Physiol Behav 38:423–426 [DOI] [PubMed] [Google Scholar]
- McLaughlin CL, Baile CA 1981 Ontogeny of feeding behavior in the Zucker obese rat. Physiol Behav 26:607–612 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thurlby PL, James WP 1977 Thermogenic defect in pre-obese ob/ob mice. Nature 266:60–62 [DOI] [PubMed] [Google Scholar]
- Yen TT, Acton JM 1972 Locomotor activity of various types of genetically obese mice. Proc Soc Exp Biol Med 140:647–650 [DOI] [PubMed] [Google Scholar]
- Dauncey MJ 1986 Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia 42:547–549 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U 1999 Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5:1066–1070 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A 1998 Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS 2005 Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 3:e415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- Korner J, Chua Jr SC, Williams JA, Leibel RL, Wardlaw SL 1999 Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology 70:377–383 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua Jr SC, Xu AW, Barsh GS, Rossetti L, Accili D 2006 Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12:534–540 [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua Jr SC, Leibel RL, Wardlaw SL 2001 Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13:959–966 [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD 2005 NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310:683–685 [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC 2005 Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8:1289–1291 [DOI] [PubMed] [Google Scholar]
- Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S 2005 Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146:1179–1191 [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua Jr SC, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 1:63–72 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Niswender KD, Rhodes CJ, Myers Jr MG, Blevins JE, Baskin DG, Schwartz MW 2003 Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky [fa(k)/fa(k)] rats. Endocrinology 144:2016–2024 [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua Jr SC 2004 An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome 15:677–685 [DOI] [PubMed] [Google Scholar]
- Kaelin CB, Gong L, Xu AW, Yao F, Hockman K, Morton GJ, Schwartz MW, Barsh GS, Mackenzie RG 2006 Stat binding sites but not Stat3 are required for fasting-induced transcription of Agouti-related protein mRNA. Mol Endocrinol 20:2591–2602 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS 2005 PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin CB, Xu AW, Lu XY, Barsh GS 2004 Transcriptional regulation of agouti-related protein (Agrp) in transgenic mice. Endocrinology 145:5798–5806 [DOI] [PubMed] [Google Scholar]
- Smith GP 2006 Ontogeny of ingestive behavior. Dev Psychobiol 48:345–359 [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Liu SM, Leibel RL, Chua Jr SC 2001 Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50:425–435 [DOI] [PubMed] [Google Scholar]
- Koch RM, Cundiff LV, Gregory KE, Van Vleck LD 2004 Genetic response to selection for weaning weight or yearling weight or yearling weight and muscle score in Hereford cattle: efficiency of gain, growth, and carcass characteristics. J Anim Sci 82:668–682 [DOI] [PubMed] [Google Scholar]
- Snowder GD, Van Vleck LD 2003 Estimates of genetic parameters and selection strategies to improve the economic efficiency of postweaning growth in lambs. J Anim Sci 81:2704–2713 [DOI] [PubMed] [Google Scholar]
- Brockmann GA, Karatayli E, Haley CS, Renne U, Rottmann OJ, Karle S 2004 QTLs for pre- and postweaning body weight and body composition in selected mice. Mamm Genome 15:593–609 [DOI] [PubMed] [Google Scholar]
- Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR 1998 Mode of action of OB protein (leptin) on feeding. Am J Physiol 275:R174–R179 [DOI] [PubMed] [Google Scholar]
- Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, Ozata M, Martin C, Walden H, Arnett C, Licinio J 2005 Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite 45:75–80 [DOI] [PubMed] [Google Scholar]
- Salorio CF, Hammond PB, Schwartz GJ, McHugh PR, Moran TH 1994 Age-dependent effects of CCK and devazepide in male and female rats. Physiol Behav 56:645–648 [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua Jr SC 2005 Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab 289:E403–E411 [DOI] [PubMed] [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML 2004 Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua Jr S, Liu SM, Li Q, Yang L, Thassanapaff VT, Fisher P 2002 Differential β cell responses to hyperglycaemia and insulin resistance in two novel congenic strains of diabetes [FVB-Lepr (db)] and obese [DBA-Lep (ob)] mice. Diabetologia 45:976–990 [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua Jr SC 2005 Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.