Abstract
Progesterone has the capacity to suppress hypothalamic dopaminergic neuronal activity on proestrous afternoon and prolong or amplify the preovulatory prolactin surge in rats. In the present study, we examined enzyme activity and phosphorylation state of tyrosine hydroxylase (TH) in the stalk-median eminence of cycling female rats on proestrus and estrus and related these to circulating progesterone levels. Phospho-TH levels were evaluated by Western blot analysis. TH activity was determined from the rate of 3,4-dihydroxyphenylalanine (DOPA) accumulation. Phospho-TH levels at Ser-19, Ser-31, and Ser-40 were similar at 1100, 1300, and 1500 h on proestrus but declined at 1700, 1900, and 2200 h, coincident with rising serum progesterone levels. Similarly, DOPA accumulation was 30–50% lower at 1700, 1900, and 2200 h as compared with 1100–1500 h on proestrus. Ser-31 and Ser-40 phosphorylation states were increased by 1100 h on estrus to a level similar to 1100 h on proestrus, whereas DOPA accumulation was 30% greater on estrous as compared with proestrous morning. There were no significant differences among the several time points on proestrus and estrus with regard to TH protein or β-tubulin levels. Exogenous progesterone administration (7.5 mg/kg, sc) before the preovulatory progesterone surge decreased TH activity and phospho-TH at Ser-19, Ser-31, and Ser-40, accompanied by premature increased serum prolactin. Our study suggests that decreased TH phosphorylation at Ser-19, Ser-31, and Ser-40 contributes to the decline in TH activity in the stalk-median eminence on proestrous afternoon and that progesterone may cause this initial cytoplasmic response of TH dephosphorylation.
DOPAMINE IS THE major factor to inhibit the secretion of prolactin from the anterior pituitary. In particular, tuberoinfundibular dopaminergic (TIDA) neurons, which originate from arcuate nucleus of the mediobasal hypothalamus and project to the stalk-median eminence (SME), provide the major dopaminergic input to the anterior pituitary (1,2). Tyrosine hydroxylase (TH) is the first and rate-limiting enzyme in the biosynthesis of catecholamines, including dopamine, norepinephrine, and epinephrine and it catalyzes the hydroxylation of l-tyrosine to 3,4-dihydroxyphenylalanine (DOPA). TH is highly regulated by both long-term mechanisms involved in transcriptional and posttranscriptional activities and short-term mechanisms accomplished by dynamic changes in the phosphorylation/dephosphorylation state of the existing enzyme (3,4,5,6). TH can be phosphorylated on four different serine residues (Ser-8, Ser-19, Ser-31, and Ser-40) in the N-terminal regulatory domain, with each serine being a target for specific protein kinases (3,6). Three of these serine residues, including Ser-19, Ser-31, and Ser-40, are regulated in vivo (7).
A number of carefully choreographed hormonal changes take place during the preovulatory period, including a rise in prolactin on the afternoon of proestrus in the rat (8). The proestrous prolactin surge has a luteolytic role in the induction of apoptosis in the regressing (nonfunctional) corpus luteum, a process necessary to maintain estrous cyclicity (9,10,11). This luteolytic effect contrasts with the luteotrophic effect on a functional corpus luteum in rodents, if a mating stimulus occurs (12). A number of studies indicate changes in TIDA neuronal activity during the estrous cycle and on proestrous day in particular (13,14,15,16,17,18,19). A decrease in TIDA neuronal activity is often associated with the broad preovulatory prolactin surge on proestrus, although the exact timing varies between laboratories (15,16,17,18,19). The rising titer of estradiol during the 24-h period before 1000 h on proestrous morning is responsible for stimulating the proestrous prolactin surge (20). Estradiol on proestrous morning without an additional ovarian input appears to be sufficient for the early phase of the prolactin surge (21). Studies in our laboratory have implicated the preovulatory progesterone rise as causal for decreased TIDA neuronal activity, which results in amplification or prolongation of the prolactin surge (21,22). A decrease in dopamine turnover rates, TH activity, and TH mRNA levels occur concomitantly with the rise in preovulatory rise in circulating progesterone (17,18,21,22). Acute ovariectomy on proestrous morning prevents this decline in TH activity and TH mRNA levels, whereas progesterone, but not estradiol, replacement at the appropriate time restores the decrease in TH function (21,22). The change in dopaminergic neuronal activity with acute ovariectomy on proestrous morning is associated with the elimination of the plateau phase or a decrease in amplitude of the later phase of preovulatory prolactin surge (21,22). Progesterone, but not estradiol, replacement at the appropriate time restores prolactin levels to values similar to those observed during the prolactin surge (21,22).
The kinetic change of reduced affinity of TH for its pterin cofactor and the ability of okadaic acid to reverse the decrease in TH activity suggest dephosphorylation of TH may play a role (18,22). Indeed, progesterone causes a rapid, but transient, decrease in TH activity in fetal hypothalamic cells in vitro, which is associated with decreased radiolabeled phosphate incorporation into TH protein (23). TH activity in the SME increases by estrous morning, even though the TH mRNA level as well as quantity of TH protein in the TIDA system are lower on estrus compared with proestrus and diestrus (22,24). Examination of the phosphorylation patterns of TH on proestrous afternoon will provide insight into the cellular signaling pathways, which may be altered by progesterone.
Our study aimed to explore the cellular mechanism underlying the inhibitory effects of progesterone upon dopamine activity in TIDA system. Specifically, the objectives include 1) to determine TH catalytic activity in the SME of cycling female rats on proestrous afternoon and estrous morning; 2) to investigate the phosphorylation state of Ser-19, Ser-31, and Ser-40 for TH in the SME of cycling female rats on proestrus and estrus; 3) to relate changes in TH function to circulating levels of progesterone, estradiol, and prolactin; and 4) to explore the reaction of TIDA neurons in a condition of exposure to an exogenous progesterone administration on proestrus.
Materials and Methods
Animals
Adult female (200–250 g) Sprague Dawley rats were obtained from Charles River (Raleigh, NC) and housed under controlled temperature and lighting (lights on from 0700–2100 h). Rats were supplied with food and water ad libitum. Estrous cycles were followed by daily vaginal lavage, and only those displaying at least three consecutive 4-d estrous cycles were used. Experiments were conducted on proestrus or estrus. All animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Southern Illinois University at Carbondale.
In experiment 1, normal cycling rats were divided into seven groups. Rats were killed at 1100, 1300, 1500, 1700, 1900, and 2200 h on proestrus and 1100 h on estrus. SME was dissected with fine scissors using a dissecting microscope and used to determine phosphorylation state of TH at Ser-19, Ser-31, and Ser-40 and TH enzyme activity. Trunk blood was collected and centrifuged at 10,000 × g for 5 min to separate serum. The serum was stored at −20 C until it was analyzed for progesterone, estradiol, and prolactin levels.
The second experiment explored whether exogenous progesterone administration before the onset of preovulatory progesterone rise on proestrus could induce a similar effect in the TIDA neurons. Two groups of rats on proestrus were used and treated with either progesterone (7.5 mg/ml·kg, sc) or oil (1 ml/kg) at 0930 h on proestrous morning and killed 5 h later at 1430 h. This dose for progesterone decreased TH mRNA level and enzyme activity in the TIDA system in our previous studies (21,22). TH phosphorylation states of Ser-19, Ser-31, and Ser-40 and TH activity in the SME of rats were evaluated, accompanied by analysis of serum prolactin levels. A separate group of rats was implanted with a chronic jugular cannula on diestrus-2 and treated with progesterone on proestrus as described above. Blood was collected via the cannula 5 h after treatment and centrifuged to collect plasma, which was analyzed for serum progesterone concentrations.
Western blot for phospho-TH and TH protein assays
Each SME tissue sample was homogenized by sonication in 35 μl homogenization buffer. The buffer contained 30 mm potassium phosphate buffer (pH 7.5), 25 mm sodium fluoride, 5 mm sodium pyrophosphate, 1 mm EDTA, aprotinin (10 μg/ml), leupeptin (10 μg/ml), pepstatin A (10 μg/ml), 500 μm phenylmethylsulfonyl fluoride, 0.5 μm okadaic acid, 20 mm sodium orthovanadate, and 1% IGEPAL CA-630. Samples were centrifuged for 15 min at 12,000 × g at 4 C and the supernatant collected. A 2.5-μl aliquot from each sample was assayed for protein content by the method of Bio-Rad protein assay. An equivalent amount of Laemmli sample buffer (Sigma-Aldrich Chemical Co., St. Louis, MO) containing 5% 2-mercaptoethanol was added to each supernatant, and samples were heated to 95 C for 4 min.
Total proteins were resolved on an 8% polyacrylamide gel slab. An equivalent amount of protein (10–25 μg) from each experimental sample was added to individual wells. For experiment 1, two samples from the 1100-h proestrous group were included on each gel and averaged to serve as beginning proestrous values. Separate gels were prepared for analysis of phospho-TH at Ser-19, Ser-31, Ser-40, and TH expression. Gels were calibrated with molecular mass standards between 49 and 211 kDa (Bio-Rad Laboratories, Hercules, CA). The separated proteins were electrophoretically transferred to a polyvinylidene difluoride membrane.
The membranes were then subjected to an immunoblot procedure. Nonspecific binding was blocked by incubating membranes with 0.2% I-Block (Tropix, Bedford, MA.) in PBS for 1 h. Membranes were incubated overnight at 4 C with one of the following antisera combinations diluted in 1% BSA-PBS-T (0.05% Tween 20 in PBS): 1) rabbit anti-phospho-TH at Ser-19 (1:2500, NE 1000 from Oncogene Research Products, San Diego, CA; or 36-9800 from Zymed Laboratories Inc., South San Francisco, CA) and mouse anti-TH (1:3000, MAB 318 from Chemicon International, Temecula, CA); 2) rabbit anti-phospho-TH at Ser-31 (1:2500, NE1001 from Calbiochem-Novabiochem Corp., San Diego, CA; or 36-9900 from Zymed) and mouse anti-TH (1:3000); and 3) rabbit anti-phospho-TH at Ser-40 (1:2500, 657016 from Calibiochem-Novabiochem; or 36-8600 from Zymed) and mouse anti-TH (1:3000); 4) rabbit anti-TH (1:3000, AB152 from Chemicon International) and mouse anti-β-tubulin (1:4000, Upstate Biotechnology). It should be noted that antisera from different commercial sources were used to detect phospho-TH signal in experiments 1 and 2, but identical results were obtained using these antisera on test blots. After incubation with primary antiserum, membranes were washed four times for 15 min each in PBS-T on an orbital shaker. Subsequently, the membranes were incubated for 40 min with both IRDye 800 conjugated affinity-purified antimouse IgG (1:20,000, 610-132-003 from Rockland Immunochemicals, Inc., Gilbertsville, PA) and Alexa Fluor 680 goat antirabbit IgG (1:20,000, A-21109 from Invitrogen Corp., Carlsbad, CA) diluted in PBS-T containing 0.02% SDS. Membranes were then washed four times for 15 min each with PBS-T. The respective proteins were detected with Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) by scanning the membranes simultaneously in the 700- and 800-nm channels. No crossover signal was observed between the two channels. Protein band intensities were quantified using the associated ArrayPro Analyzer 4.5 Software. Each phospho-TH band detected in the 700-nm channel was normalized to its respective TH signal detected in the 800-nm channel for individual blots. For analysis of total TH quantity, TH band signals detected in the 700-nm channel were normalized to the respective β-tubulin signals detected in the 800-nm channel. The normalization corrected for any variations in loading and transfer efficiency. For experiment 1, two independent samples from the 1100-h proestrous group and one sample from each of the other experimental groups were included on individual blots. Data from individual blots were expressed as the percent change for the 1100-h sample, and data from nine blots were averaged to obtain final data. For experiment 2, all samples were included on two blots. Control samples on each blot were averaged and data expressed as percent control.
Estimation of TH activity by HPLC
Rats were injected with m-hydroxybenzylhydrazine dihydrochloride (NSD 1015; 100 mg/kg, ip), an l-aromatic amino acid decarboxylase inhibitor, 30 min before killing the rats, as described previously (22). The SME was dissected and homogenized by sonication in 250 μl 0.1 n perchloric acid and centrifuged at 13,000 × g for 15 min. The tissue content of DOPA in the supernatant was determined by HPLC with electrochemical detection, as described previously (22,25). The pellet was solubilized in 0.5 n sodium hydroxide and analyzed for protein content by the method of Bio-Rad protein assay. Tissue DOPA levels were normalized to protein contents.
Serum progesterone, estradiol, and prolactin levels detected by RIA
Serum progesterone concentrations were measured using Coat-A-Count kit (Diagnostic Products Corp., Los Angeles, CA) with a sensitivity of 0.02 ng/ml. The intra- and interassay coefficients of variation were 7.5 and 11.6%, respectively. Serum estradiol concentrations were measured using a double-antibody kit (Diagnostic Products) with a sensitivity of 1.4 pg/ml. The intra- and interassay coefficients of variation were 4.2 and 14.4%, respectively. Serum prolactin levels were assessed using a RIA kit provided by Dr. Albert Parlow and the National Hormone and Pituitary Program. Prolactin RP-3 was used as a reference preparation, and the limit of sensitivity for the assay was 0.25 ng/ml. The intra- and interassay coefficients of variation were 12.9 and 8.4%, respectively.
Statistical analysis
The results are expressed as the mean ± sem. Data were evaluated by one-way ANOVA, and multiple comparisons were made with Fisher’s least significant procedures for experiment 1. Student’s t test was used for the comparison between progesterone- and vehicle oil-treated groups in experiment 2. P < 0.05 was considered a statistically significant difference.
Results
Phosphorylation state of TH in the SME of cycling female rats
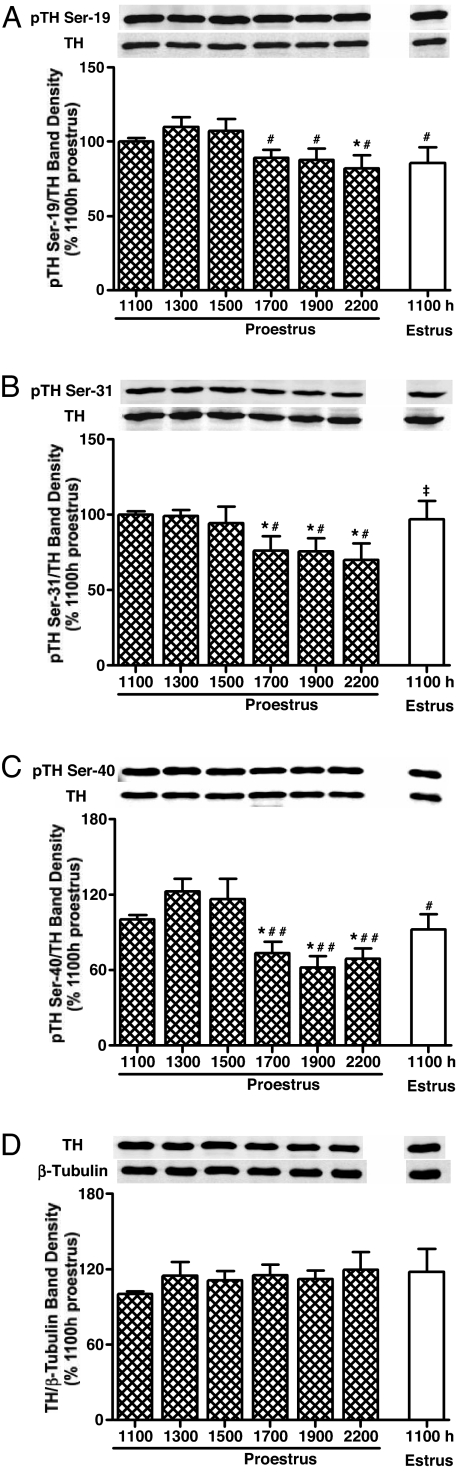
TH phosphorylation states at Ser-19, Ser-31, and Ser-40 in the SME of rats were evaluated at 1100, 1300, 1500, 1700, 1900, and 2200 h on proestrus and 1100 h on estrus. A similar profile of TH dephosphorylation was observed on the late afternoon of proestrus for Ser-19, Ser-31, and Ser-40 (Fig. 1). Phospho-TH at Ser 19 values were similar on proestrus at 1100, 1300, and 1500 h but modestly declined 16–23% at 1700–2200 h (Fig. 1A). Phospho-TH at Ser-31 values were also similar between 1100 and 1500 h on proestrus and were moderately reduced by 22–28% on proestrous afternoon between 1700 and 2200 h (Fig. 1B). Phospho-TH at Ser-40 values were similar at 1100, 1300, and 1500 h on proestrus but markedly declined by 30–50% at 1700, 1900, and 2200 h (Fig. 1C). Ser-31 and Ser-40 phosphorylation states were increased by 1100 h on estrus to a level similar to 1100 h on proestrus, whereas Ser-19 values remained low similar to 2200 h on proestrus (Fig. 1, A–C). There is no significant difference among the time points with regard to TH protein or β-tubulin levels (Fig. 1D).
Figure 1.
Phosphorylation states of Ser-19 (A), Ser-31 (B), and Ser-40 (C) for TH and total TH protein (D) in the SME of cycling female rats at designated time points on proestrus and estrus. Phospho-TH at Ser-19, Ser-31, and Ser-40 signal levels were decreased at 1700, 1900, and 2200 h compared with the morning of proestrus. Phosphorylation states of Ser-31 and Ser-40, but not Ser-19, were increased at 1100 h on estrus. Phospho-TH values were individually normalized to respective TH values. TH values were individually normalized to respective β-tubulin values. Each value represents a mean ± sem of determinations from nine individual blots representing nine to 18 (1100 h proestrous only) rats. *, P < 0.01 vs. 1100 h on proestrus; #, P < 0.05 vs. 1300 and 1500 h on proestrus; ##, P < 0.01 vs. 1300 and 1500 h on proestrus; ‡, P < 0.05 vs. 1700–2200 h on proestrus.
TH activity in the SME of cycling female rats
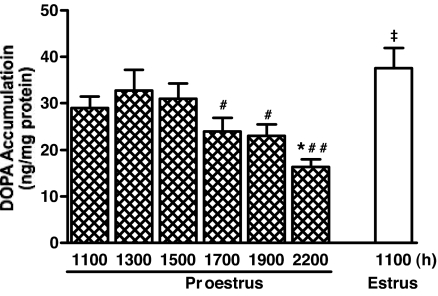
TH activity as evaluated by DOPA accumulation in the SME of cycling female rats were similar at 1100, 1300, and 1500 h on proestrus and was 30–50% lower at 1700, 1900, and 2200 h compared with 1100–1500 h on proestrus (Fig. 2). DOPA accumulation was 30% greater at 1100 h on estrus compared with that of 1100 h on proestrous morning.
Figure 2.
TH activity as evaluated by DOPA accumulation in the SME of cycling female rats at designated time points on proestrus and estrus. TH activity was 30–50% lower at 1700, 1900, and 2200 h compared with 1100–1500 h on proestrus, but 30% greater on estrous compared with proestrous morning. Each value represents mean ± sem of determinations from seven to nine rats. *, P < 0.05 vs. 1100 h on proestrus; #, P < 0.05 vs. 1300 and 1500 h on proestrus; ##, P < 0.01 vs. 1300 and 1500 h on proestrus; ‡, P < 0.05–0.001 vs. 1100, 1700–2200 h on proestrus.
Serum progesterone, estradiol, and prolactin levels in cycling female rats
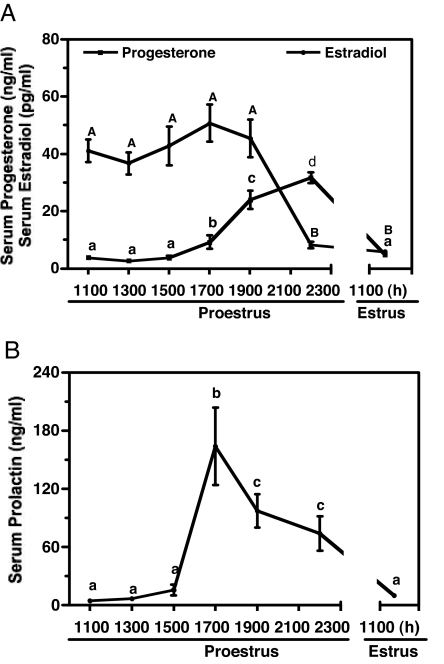
Serum progesterone, estradiol, and prolactin concentrations at designated time points on proestrus and estrus were examined to establish a physiological basis to relate to the phosphorylation state and activity of TH in the SME of rats. Circulating progesterone levels were low at 1100–1500 h on proestrus and elevated about 3-, 7-, and 9-fold at 1700, 1900, and 2200 h on proestrus, respectively (Fig. 3A). Progesterone concentrations in serum were reduced to basal values on the morning of estrus. Serum estradiol levels were high from 1100–1900 h on proestrus, whereas they declined to one fifth of the value at 2200 h on proestrus and remained at a low level at 1100 h on estrus (Fig. 3A). Serum prolactin levels were low at 1100–1500 h and reached a peak value at 1700 h on proestrus, which is 18-fold higher than that of 1100–1500 h. Serum prolactin concentrations maintained at 11- and 8-fold higher values at 1900 h and 2200 h, respectively, compared with that of proestrous morning and decreased to a level similar to that of proestrous morning at 1100 h on estrus (Fig. 3B).
Figure 3.
Circulating progesterone and estradiol concentrations (A) and prolactin concentrations (B) in rats on proestrus and estrus. Circulating progesterone levels were elevated (P < 0.05–0.001) at 1700, 1900, and 2200 h on proestrus. Serum estradiol levels were increased (P < 0.001) at 1100–1900 h on proestrus. Serum prolactin levels were elevated (P < 0.05–0.001) at 1700 h on proestrus and maintained a high value at 1900 and 2100 h. Each value is a mean ± sem of determinations from six to 13 rats. Different letters represents statistically significant differences among the groups.
Experiment 2: phosphorylation state of TH in the SME of progesterone-treated rats on proestrus
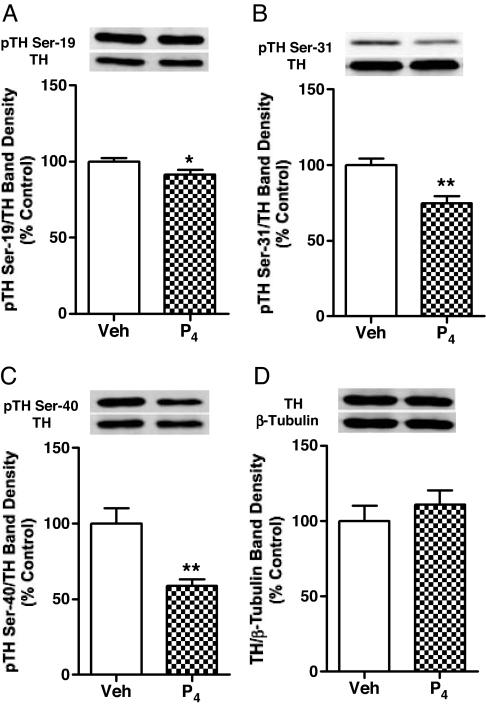
Progesterone administration at a dose of 7.5 mg/kg (sc) at 0930 h on proestrus resulted in circulating progesterone levels of 75 ng/ml at 1430 h and induced a significant decrease in TH phosphorylation signal levels at Ser-19, Ser-31, and Ser-40 by 5 h later, as shown in Fig. 4, A–C. The magnitude of change at each specific serine was similar to values at 2200 h on proestrus, approximately 5 h after the onset of the endogenous progesterone rise. The decrease in Ser-40 phosphorylation signal was most dramatic to 50% of control levels, whereas Ser-31 phosphorylation was decreased by 30% and Ser-19 was decreased by 20%. Rats were killed at 1430 h, a time point before the preovulatory progesterone surge, when progesterone levels were still low in vehicle-treated rats. TH protein or β-tubulin levels in the SME of rats were not affected by progesterone treatment (Fig. 4D).
Figure 4.
Phosphorylation states of Ser-19 (A), Ser-31 (B), and Ser-40 (C) for TH and total TH protein quantity (D) in the SME of cycling female rats. Rats were injected with progesterone (7.5 mg/kg, sc) or vehicle oil on proestrous morning (0930 h) and killed 5 h later. Phospho-TH values were individually normalized to respective TH values. TH protein values were individually normalized to respective β-tubulin values. Data are expressed as mean ± sem of determinations from eight rats. *, P < 0.05; **, P < 0.01 vs. control.
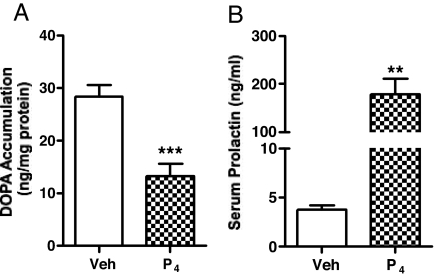
TH activity in the SME of progesterone-treated rats
Consistent with our data on phosphorylation state of TH after progesterone administration in the cycling rats on proestrus, TH activity as evaluated by DOPA accumulation in the SME was significantly lower in the progesterone-treated vs. oil-treated rats, with 50% lower values than that of oil-treated group (Fig. 5A). The magnitude of change was similar to that on proestrus at 2200 h approximately 5 h after the onset of the endogenous progesterone rise (Fig. 2).
Figure 5.
TH activity (A) as evaluated by DOPA accumulation in the SME, and serum prolactin levels (B) in proestrous rats treated with progesterone. Rats were treated with progesterone (7.5 mg/kg, sc) or vehicle oil on proestrous morning (0930 h) and killed 5 h later. Data represents mean ± sem of determinations from six to eight rats. **, P < 0.01; ***, P < 0.001 vs. control.
Serum prolactin level in progesterone-treated rats
Serum prolactin levels in the progesterone-treated rats were significantly increased 5 h after progesterone administration compared with that of vehicle-treated group. The prolactin level in the progesterone-treated rats is about 50-fold higher than that of the control (Fig. 5B) and 2-fold higher than that at 2200 h on proestrus (Fig. 3B).
Discussion
The major finding of this study is that progesterone has the capacity to dephosphorylate specific serine residues of TH in the SME of rats and inhibit TH enzyme activity. Decreased phospho-TH at Ser-19, Ser-31, and Ser-40 along with a decline in TH activity suggests that dephosphorylation of these three regulatory serine residues in TH may acutely mediate the inhibitory effect on the catalytic activity of TH. Exogenous progesterone administration before the onset of preovulatory progesterone surge on proestrous afternoon caused dephosphorylation of TH at Ser-19, Ser-31, and Ser-40 and decreased TH activity in the SME in a manner similar to events during the endogenous progesterone surge. These data support the notion that progesterone may mediate an effect in the cytoplasm of TIDA neurons typically associated with second messenger signaling pathways.
TH can be phosphorylated at four serine residues in vitro and in situ by a variety of protein kinases. Three serine residues, Ser-19, Ser-31, and Ser-40, can be phosphorylated in vivo (7), although relatively little is known about roles for specific serine phosphorylation sites in response to physiologically relevant stimuli, particularly in TIDA neurons. As reviewed by Dunkley et al. (6), Ser-40 serves as a phosphorylation site of multiple protein kinases including protein kinase A, protein kinase G, and protein kinase C. Ser-19 is a substrate for calcium- and calmodulin-stimulated protein kinase II and MAPK-activated protein kinase 2. ERK-1 and -2 are the only kinases identified to phosphorylate Ser-31. Our data indicate that progesterone caused dephosphorylation of all three of these major regulatory phosphorylation sites in the N-terminal regulatory domain of TH, suggesting that multiple intracellular signaling pathways may be modified as a result of progesterone treatment.
Progesterone may also modulate the activity of a phosphatase enzyme active at the three phosphorylation sites. Although the contribution of phosphatases to the cellular regulation of TH are less studied compared with the protein kinase counterparts, this mechanism may account for the concerted dephosphorylation of all three serines. Indeed, Ser-19, Ser-31, and Ser-40 are targets for protein phosphatase 2A (6,26), whereas protein phosphatase 2C acts on Ser-19 and Ser-40 (27,28). Our previous data demonstrating that okadaic acid, a protein phosphatase-2A and -1 inhibitor, reverses the dephosphorylation at 2200 h on proestrus supports the notion that phosphatase regulation may play a role (22). However, the differential impact on specific serine residues with respect to the magnitude of the change suggests a complex interplay in modulation of the kinase and phosphatase activity.
TH phosphorylation at Ser-31 and Ser-40 are directly involved in the activation of TH (29,30). In general, phosphorylation of Ser-40 appears to exert the major effect on TH catalytic activity, because phosphorylation of TH at Ser-40 can activate TH by up to 20-fold (29), whereas Ser-31 produces a less than 2-fold increase in TH activity (31). Phosphorylation of TH at the Ser-40 site by cAMP-dependent protein kinase alters the kinetic properties of TH by increasing the affinity of TH for its pterin cofactor (32,33). Phosphorylation of Ser-19 does not directly increase TH activity either in vitro or in vivo, although the activity of TH phosphorylated by multifunctional calcium- and calmodulin-dependent protein kinase II may be increased in the presence of 14-3-3 activator protein (34,35). A major role of Ser-19 phosphorylation may be in potentiating phospho-TH at Ser-40 and subsequent activation of TH (28,36,37,38). In the current study, the most marked changes occurred at the Ser-40 phosphorylation site on proestrous afternoon. These data are consistent with our previous data that the apparent Km of TH for the pterin cofactor was increased in the SME at 2200 h during the late evening preovulatory period (18). This kinetic effect was reversible with cAMP-dependent activation (18). The decreased affinity of TH for its pterin cofactor is consistent with regulation at the Ser-40 site as the major factor impacting TH activity. The marked decline in Ser-40 phosphorylation state is also consistent with the observed suppression in TH activity, given that the state of this phosphorylation site seems to exert more impact on TIDA neuronal activity.
The phosphorylation states of TH at Ser-19, Ser-31, and Ser-40 were decreased concomitant with the circulating progesterone rise at 1700, 1900, and 2200 h on proestrus. Ser-31 and Ser-40 phosphorylation state recovered to a level similar to that on proestrous morning when serum progesterone declined on estrus, whereas the Ser-19 phosphorylation state stayed low. Our data demonstrate that progesterone has the capacity to dephosphorylate TH at Ser-19, Ser-31, and Ser-40 when rats were treated with progesterone before the preovulatory progesterone rise on proestrus. Interestingly, the magnitude of change for the specific serine residues was similar with exogenous progesterone treatment compared with the afternoon of proestrus concomitant with the endogenous progesterone rise. Our previous studies support the notion that progesterone is responsible for the late afternoon decline in TH activity during the preovulatory or proestrous day (21,22). Acute ovariectomy at 1200 h and thus removal of the source of progesterone, prevents the decline in TH activity, whereas progesterone, but not estradiol, administration at the appropriate times restored the decrease (21,22). Yen and Pan (39) provided additional evidence that progesterone advances the diurnal rhythm in estradiol-treated ovariectomized rats and further reported that the effect on TIDA neuronal activity was blocked with RU486 (progesterone/glucocorticoid receptor antagonist) treatment, intracerebroventricular injections of antisense oligonucleotides to the progesterone receptor, and progesterone receptor antibody administration. However, the ability of RU486 to block progesterone-induced suppression of TIDA neuronal activity and increased prolactin levels is dependent on administration for at least 2 d before progesterone exposure (39). When RU486 is administered on the morning of proestrus, it is ineffective in blocking progesterone’s effect on TIDA neuronal activity (22), even though this paradigm of RU486 administration with respect to timing and dose effectively disrupted other progesterone-dependent events on proestrus, such as the induction of fos expression in GnRH neurons (40).
The acute inhibitory effect of progesterone appears to occur, at least in part, by an intrahypothalamic site of action. Progesterone induces a decrease in radiolabeled phosphate incorporation in TH and suppression of TH activity in rat fetal hypothalamic cells in vitro when extrahypothalamic inputs are removed (23). However, the specific serine residues that progesterone targets were not identified in this earlier in vitro study. As shown in this study, decreased TH activity in the SME of cycling female rats as related to progesterone rise on proestrous afternoon is coincident with decreased phospho-TH at Ser 19, Ser-31, and Ser-40, suggesting that dephosphorylation of TH modulates this acute inhibitory effect on the TH activity in response to a physiological hormonal stimulus.
Ovarian steroids estradiol and progesterone interact to influence the activity of neuroendocrine dopaminergic neurons and prolactin secretion (1,2). However, the mechanism(s) of steroid hormone action and interactions between estradiol and progesterone appear to be complex and may be different in magnitude and direction dependent on concentration and timing. Progesterone can be either inhibitory or stimulatory to TIDA neuronal activity. An early inhibitory response, which occurs at 1700 h within 1 h of the endogenous progesterone rise on proestrus (this study) and transiently in hypothalamic cells in vitro 1–6 h after progesterone treatment (23), involves dephosphorylation of TH protein. A later response of proestrus at 2200 h, but not 1700 h, and extending into estrous morning is a decrease in cellular TH mRNA signal levels (22). A similar decrease in the number of detectable TH mRNA is observed in steroid-treated ovariectomized rats at 8 h, but not 2 h, after progesterone administration (41). The inhibitory effect on TH gene expression was not observed in vitro (23), which suggests that progesterone’s effect on TH phosphorylation state and TH gene expression may be mediated by different mechanisms. Even though progesterone has this inhibitory effect on TH mRNA levels (22,41), TH quantity was similar throughout the day on proestrus to estrous morning in the present study. The relatively long half-life for TH protein may account for the apparently disconnected findings. The half-life is about 30 h in chromaffin cells and pheochromocytoma cell lines under basal conditions (42,43) and can be 3–8 d in vivo (44,45). The long-term effects of concomitant treatment with estradiol and progesterone results in an increase in TH mRNA signal levels in the arcuate nucleus, indicating that with prolonged steroid hormone treatment progesterone antagonizes the inhibitory effects of estradiol treatment (46).
A number of studies demonstrate that the action of progesterone require previous treatment with estradiol (46,47,48,49,50). Estradiol can up-regulate progesterone receptor expression in the hypothalamus (51,52), which may provide the environment for progesterone to exert this inhibitory effect. Colocalization of classical nuclear estrogen and/or progesterone receptors with dopamine in hypothalamus (53,54,55) provides an anatomical basis for the interactions between steroids and tuberoinfundibular dopamine. Although a genomic mechanism for this progesterone action cannot be discounted, a more rapid action for progesterone is a more attractive hypothesis. The cytoplasmic localization of progesterone receptor, particularly the B isoform, in some cell lines using green fluorescent protein chimeras supports a potential mechanism for cytoplasmic action (56). Another line of evidence supporting nongenomic actions for the classical progesterone receptor, particularly the B isoform, are reports of interactions with cytoplasmic signaling pathways (57). Alternatively, the identification of novel membrane progesterone receptors and interactions of progesterone metabolites with GABA-A receptors provide additional avenues for progesterone to alter neuronal function at the membrane level (58,59), because GABA regulates hypothalamic dopamine function (60,61,62). Membrane progesterone receptor subtypes in other cell types have been associated with coupling to Gi proteins, resulting in decreased cellular cAMP concentrations (63). Progesterone acting on a membrane receptor coupled to a Gi protein is an attractive hypothesis, given that the most dramatic effects are observed with Ser-40 dephosphorylation and that Ser-40 is a target for cAMP-dependent protein kinase. Identification of progesterone receptor subtypes, which may be involved in this novel cytoplasmic action of progesterone on TH dephosphorylation, is an important area for further investigation.
The hormonal profile on proestrus is complex with increases in circulating levels of estradiol, progesterone, prolactin, LH, and FSH. These hormonal events elicit or are driven by complex interactions within the hypothalamus and modifications of function of many neuronal groups. The short-loop feedback of prolactin to increase TIDA neuronal activity and thus regulate prolactin secretion is well established, although the cellular signals and molecular mechanism are still not well understood. Prolactin increases the phosphorylation state of Ser-19, Ser-31, and Ser-40 in hypothalamic cells in vitro (64), and this action occurs within a time frame similar to the events on proestrus afternoon. A rapid stimulatory action of prolactin in vivo has also been described (65,66), although less complex models were used to establish this phenomenon. Cross-talk between the prolactin and progesterone signaling pathways has been observed in other tissues, and progesterone appears to be inhibitory to prolactin actions within the mammary gland (67). It is unclear whether there is an interaction between prolactin and progesterone with regard to TIDA activity on the afternoon of proestrus. Indeed, it is notable that TH activity and the TH phosphorylation state of Ser-31 and Ser-40 are increased when progesterone has declined by estrous morning, possibly due to the action of prolactin no longer opposed by progesterone.
Our study indicates that progesterone mediates TH dephosphorylation, especially on Ser-40, which leads to a decrease in TH activity. The consequence of these molecular events in the TIDA neurons is to extend or amplify the preovulatory prolactin surge on proestrous afternoon. These findings represent a novel action of progesterone to decrease the phosphorylation state of a cytoplasmic protein involved in important neurotransmitter synthesis within a physiological situation. The progesterone receptor subtype and its mechanisms of action to elicit these changes will be important areas for future investigation.
Acknowledgments
We thank Dr. A. F. Parlow, Harbor-UCLA Medical Center, and the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, for providing prolactin RIA reagents.
Footnotes
This work was support by National Institutes of Health Grants HD045805 and HD048925 to L.A.A.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 20, 2007
Abbreviations: DOPA, 3,4-Dihydroxyphenylalanine; PBS-T, 0.05% Tween 20 in PBS; SME, stalk-median eminence; TH, tyrosine hydroxylase; TIDA, tuberoinfundibular dopaminergic.
References
- Ben-Jonathan N, Hnasko R 2001 Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE 1996 Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 67:443–462 [DOI] [PubMed] [Google Scholar]
- Masserano JM, Weiner N 1983 Tyrosine hydroxylase regulation in the central nervous system. Mol Cell Biochem 53–54:129–152 [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR 1989 Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci 12:415–461 [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW 2004 Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91:1025–1043 [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA 1991 Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem 266:5650–5657 [PubMed] [Google Scholar]
- Smith MS, Freeman ME and Neill JD 1975 The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226 [DOI] [PubMed] [Google Scholar]
- Gaytán F, Bellido C, Morales C, Sánchez-Criado 1998 Both prolactin and progesterone in proestrus are necessary for the induction of apoptosis in the regressing corpus luteum of the rat. Biol Reprod 59:1200–1206 [DOI] [PubMed] [Google Scholar]
- Bowen JM, Towns R, Warren JS, Keyes PL 1999 Luteal regression in the normally cycling rat: apoptosis, monocyte chemoattractant protein-1 and inflammatory cell involvement. Biol Reprod 60:740–746 [DOI] [PubMed] [Google Scholar]
- Gaytán F, Bellido C, Morales C, Sánchez-Criado 2001 Cyclic changes in the responsiveness of regressing corpora lutea to the luteolytic effects of prolactin in rats. Reproduction 122:411–417 [DOI] [PubMed] [Google Scholar]
- Hilliard J 1973 Corpus luteum function in guinea pigs, hamsters, rats, mice and rabbits. Biol Reprod 8:203–221 [Google Scholar]
- Ben-Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC 1977 Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology 100:452–458 [DOI] [PubMed] [Google Scholar]
- Demarest KT, Johnston CA, Moore KE 1981 Biochemical indices of catecholaminergic neuronal activity in the median eminence during the estrous cycle of the rat. Neuroendocrinology 32:24–27 [DOI] [PubMed] [Google Scholar]
- Carr LA, Voogt JL 1988 Catecholamine synthesis enzymes in the hypothalamus during the estrous cycle. Brain Res 196:437–445 [DOI] [PubMed] [Google Scholar]
- Rance N, Wise PM, Selmanoff MK, Barraclough CA 1981 Catecholamine turnover rates in discrete hypothalamic areas and associated changes in median eminence luteinizing hormone-releasing hormone and serum gonadotropins on proestrus and diestrous day 1. Endocrinology 108:1795–1802 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Murai I, Ben-Jonathan N 1989 Differential alterations in dopamine turnover rates in the stalk-median eminence and posterior pituitary during the preovulatory prolactin surge. Neuroendocrinology 49:525–530 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Ben-Jonathan N 1989 Tyrosine hydroxylase in the stalk-median eminence and posterior pituitary is inactivated only during the plateau phase of the preovulatory prolactin surge. Endocrinology 125:667–674 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 1998 Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67:377–383 [DOI] [PubMed] [Google Scholar]
- Neill JD, Freeman ME, Tillson SA 1971 Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology 89:1448–1453 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Ben-Jonathan N 1990 The preovulatory prolactin surge is prolonged by a progesterone-dependent dopaminergic mechanism. Endocrinology 126:246–252 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1994 Progesterone suppresses tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nucleus on proestrus. Endocrinology 135:343–350 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 2002 Progesterone induces dephosphorylation and inactivation of tyrosine hydroxylase in rat hypothalamic dopaminergic neurons. Neuroendocrinology 75:273–281 [DOI] [PubMed] [Google Scholar]
- Porter JC 1986 Relationship of age, sex, and reproductive status to the quantity of tyrosine hydroxylase in the median eminence and superior cervical ganglion of the rat. Endocrinology 118:1426–1432 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1991 Hyperprolactinemia increases and hypoprolactinemia decreases tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nuclei, but not the substantia nigra or zona incerta. Endocrinology 128:997–1005 [DOI] [PubMed] [Google Scholar]
- Haavik J, Schelling DL, Campbell DG, Andersson KK, Flatmark, T, Cohen P 1989 Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett 251:36–42 [DOI] [PubMed] [Google Scholar]
- Bevilaqua LR, Cammarota M, Dickson PW, Sim AT, Dunkley PR 2003 Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J Neurochem 85:1368–1373 [DOI] [PubMed] [Google Scholar]
- Bevilaqua LR, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW 2001 Phosphorylation of Ser(19) alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of Ser(40). J Biol Chem 276:40411–40416 [DOI] [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF 1992 Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem 267:12639–12646 [PubMed] [Google Scholar]
- Ribeiro P, Wang Y, Citron BA, Kaufman S 1992 Regulation of recombinant rat tyrosine hydroxylase by dopamine. Proc Natl Acad Sci USA 89:9593–9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG 1992 ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci USA 89:2365–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames MM, Lerner P, Lovenberg W 1978 Tyrosine hydroxylase activation of protein phosphorylation and end product inhibition. J Biol Chem 253:27–31 [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR 1986 Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem 261:10489–10492 [PubMed] [Google Scholar]
- Itagaki C, Isobe T, Taoka M, Natsume T, Nomura N, Horigome T, Omata S, Ichinose H, Nagatsu T, Greene LA, Ichimura T 1999 Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 38:15673–15680 [DOI] [PubMed] [Google Scholar]
- Atkinson J, Richtand N, Schworer C, Kuczenski R, Soderling T 1987 Phosphorylation of purified rat striatal tyrosine hydroxylase by Ca2+/calmodulin-dependent protein kinase II: effect of an activator protein. J Neurochem 49:1241–1249 [DOI] [PubMed] [Google Scholar]
- Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M 1998 Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site- and phosphospecific antibodies and site-directed mutagenesis. J Neurochem 71:1670–1675 [DOI] [PubMed] [Google Scholar]
- Toska K, Kleppe R, Armstrong CG, Morrice NA, Cohen P, Haavik J 2002 Regulation of tyrosine hydroxylase by stress-activated protein kinases. J Neurochem 83:775–783 [DOI] [PubMed] [Google Scholar]
- Bobrovskaya L, Dunkley PR, Dickson PW 2004 Phosphorylation of Ser19 increases both Ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J Neurochem 90:857–864 [DOI] [PubMed] [Google Scholar]
- Yen SH, Pan JT 1998 Progesterone advances the diurnal rhythm of tuberoinfundibular dopaminergic neuronal activity and the prolactin surge in ovariectomized, estrogen-primed rats and in intact proestrous rats. Endocrinology 139:1602–1609 [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE 1990 Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127:2604–2606 [DOI] [PubMed] [Google Scholar]
- Morrell JI, Rosenthal MF, McCabe JT, Harrington CA, Chikaraishi DM, Pfaff DW 1989 Tyrosine hydroxylase mRNA in the neurons of the tuberoinfundibular region and zona incerta examined after gonadal steroid hormone treatment. Mol Endocrinol 3:1426–1433 [DOI] [PubMed] [Google Scholar]
- Fernández E, Craviso GL 1999 Protein synthesis blockade differentially affects the degradation of constitutive and nicotinic receptor-induced tyrosine hydroxylase protein levels in isolated chromaffin cells. J Neurochem 73:169–178 [DOI] [PubMed] [Google Scholar]
- Tank AW, Ham L, Curella P 1986 Induction of tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: effect of the inducing agents alone or in combination on the enzyme levels and rate of synthesis of tyrosine hydroxylase. Mol Pharmacol 30:486–496 [PubMed] [Google Scholar]
- Kvetnansky R, Weise VK, Kopin IJ 1970 Elevation of adrenal tyrosine hydroxylase and phenyl-N-methyltransferase by repeated immobilization of rats. Endocrinology 87:744–749 [DOI] [PubMed] [Google Scholar]
- Mueller RA, Thoenen H, Axelrod J 1969 Inhibition of transsynaptically increased tyrosine hydroxylase by cycloheximide and actinomycin D. Mol Pharmacol 5:463–468 [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1993 Progesterone reverses the estradiol-induced decrease in tyrosine hydroxylase mRNA levels in the arcuate nucleus. Neuroendocrinology 58:501–510 [DOI] [PubMed] [Google Scholar]
- Caligaris L, Astrada JJ, Taleisnik S 1974 Oestrogen and progesterone influence on the release of prolactin in ovariectomized rats. J Endocrinol 60:205–215 [DOI] [PubMed] [Google Scholar]
- Beattie CW, Rodgers CH, Soyka LF 1972 Influence of ovariectomy and ovarian steroids on hypothalamic tyrosine hydroxylase activity in the rat. Endocrinology 91:276–279 [DOI] [PubMed] [Google Scholar]
- Rance N, Wise PM, Barraclough CA 1981 Negative feedback effects of progesterone correlated with changes in hypothalamic norepinephrine and dopamine turnover rates, median eminence luteinizing hormone-releasing hormone, and peripheral plasma gonadotropins. Endocrinology 108:2194–2199 [DOI] [PubMed] [Google Scholar]
- Gonzalez HA, Kedzierski W, Aguila-Mansilla N, Porter JC 1989 Hormonal control of tyrosine hydroxylase in the median eminence: demonstration of a central role for the pituitary gland. Endocrinology 124:2122–2127 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology 138:5476–5484 [DOI] [PubMed] [Google Scholar]
- Scott REM, Wu-Peng XS, Pfaff DW 2002 Regulation and expression of progesterone receptor mRNA isoforms A and B in the male and female rat hypothalamus and pituitary following oestrogen treatment. J Neuroendocrinol 14:175–183 [DOI] [PubMed] [Google Scholar]
- Sar M 1988 Distribution of progestin-concentrating cells in rat brain: colocalization of [3H]ORG.2058, a synthetic progestin, and antibodies to tyrosine hydroxylase in hypothalamus by combined autoradiography and immunocytochemistry. Endocrinology 123:1110–1118 [DOI] [PubMed] [Google Scholar]
- Sar M 1984 Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science 223:938–940 [DOI] [PubMed] [Google Scholar]
- Fox SR, Harlan RE, Shivers BD, Pfaff DW 1990 Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology 51:276–283 [DOI] [PubMed] [Google Scholar]
- Lim CS, Baumann CT, Htun H, Xian W, Irie M, Smith CL, Hager GL 1999 Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol Endocrinol 13:366–375 [DOI] [PubMed] [Google Scholar]
- Edwards DP 2005 Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67:335–375 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE 2007 Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M 2001 Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol 22:69–106 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Moore KE, Lookingland KJ 1994 Neurochemical evidence that AMPA receptor-mediated tonic inhibition of hypothalamic dopaminergic neurons occurs via activation of inhibitory interneurons. Brain Res 660:319–322 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Goudreau JL, Moore KE, Lookingland KJ 1994 GABAergic regulation of tuberoinfundibular dopaminergic neurons in the male rats. Brain Res 659:194–200 [DOI] [PubMed] [Google Scholar]
- Lee TY, Pan JT 2001 Involvement of central GABAergic neurons in basal and diurnal changes of tuberoinfundibular dopaminergic neuronal activity and prolactin secretion. Life Sci 68:1965–1975 [DOI] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P 2006 Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol 20:1519–1534 [DOI] [PubMed] [Google Scholar]
- Ma FY, Grattan DR, Goffin V, Bunn SJ 2005 Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: the differential role of specific protein kinases. Endocrinology 146:93–102 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Lerant AA, Freeman ME 1999 Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats. Brain Res 837:236–241 [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Guibert B, Frain O, Leviel V 1994 Evidence for protein kinase C involvement in the short-term activation by prolactin of tyrosine hydroxylase in tuberoinfundibular dopaminergic neurons. J Neurochem 62:967–977 [DOI] [PubMed] [Google Scholar]
- Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, Rosen JM, Watkin H, Anderson SM, Edwards DP 2007 Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol Endocrinol 21:106–125 [DOI] [PubMed] [Google Scholar]