Fig. 1.
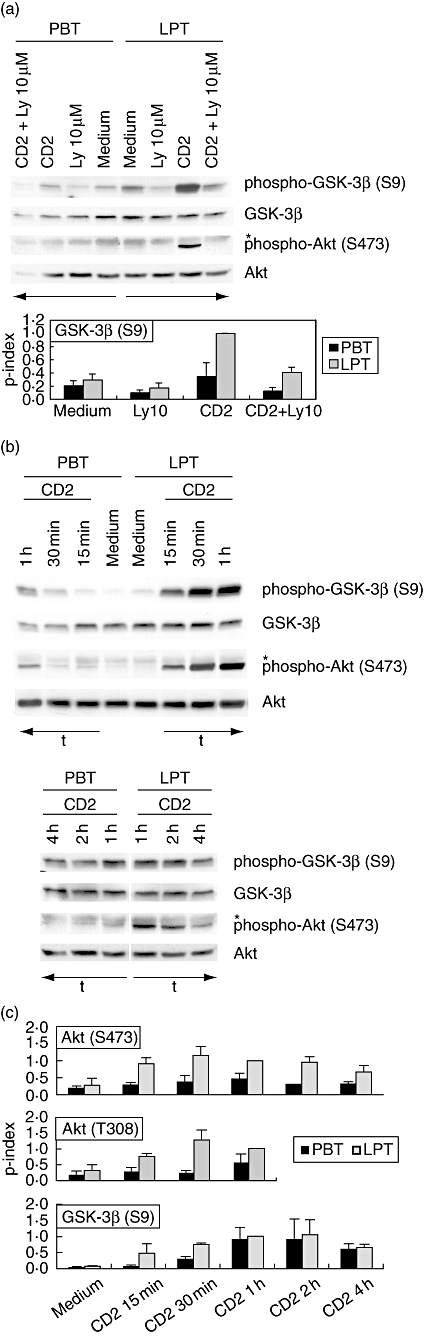
Enhanced CD2-induced phosphorylation of protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3β) in lamina propria T lymphocytes (LPT). (a) CD4+ peripheral blood T lymphocytes (PBT) and LPT were cultured in the presence or absence of CD2 monoclonal antibody (mAb) M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for 15 min. Ly294002 was added 30 min prior to stimulation. The phosphorylation state of GSK-3β (Ser9) and AKT (Ser473) was determined by immunoblotting of whole cell extracts. Results are representative of at least three independent experiments. *Non-specific band. The phosphorylation index of GSK-3β (Ser9) (lower panel) was determined as described in Materials and methods. Data are expressed as mean ± standard deviation (s.d.) (n = 3). (b) CD4+ PBT and LPT were cultured in the presence or absence of CD2 mAb M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for the indicated time-periods. The phosphorylation state of GSK-3β (Ser9) and AKT (Ser473) was determined by immunoblotting of whole cell extracts. Results are representative of at least three independent experiments. *Non-specific band. (c) Densitometric determination of the phosphorylation index of AKT (Ser473 and Thr308; upper panels) and GSK-3β (Ser9; lower panel) in CD4+ PBT and LPT in response to CD2 activation. Data are expressed as mean ± s.d. (n = 2–8).
