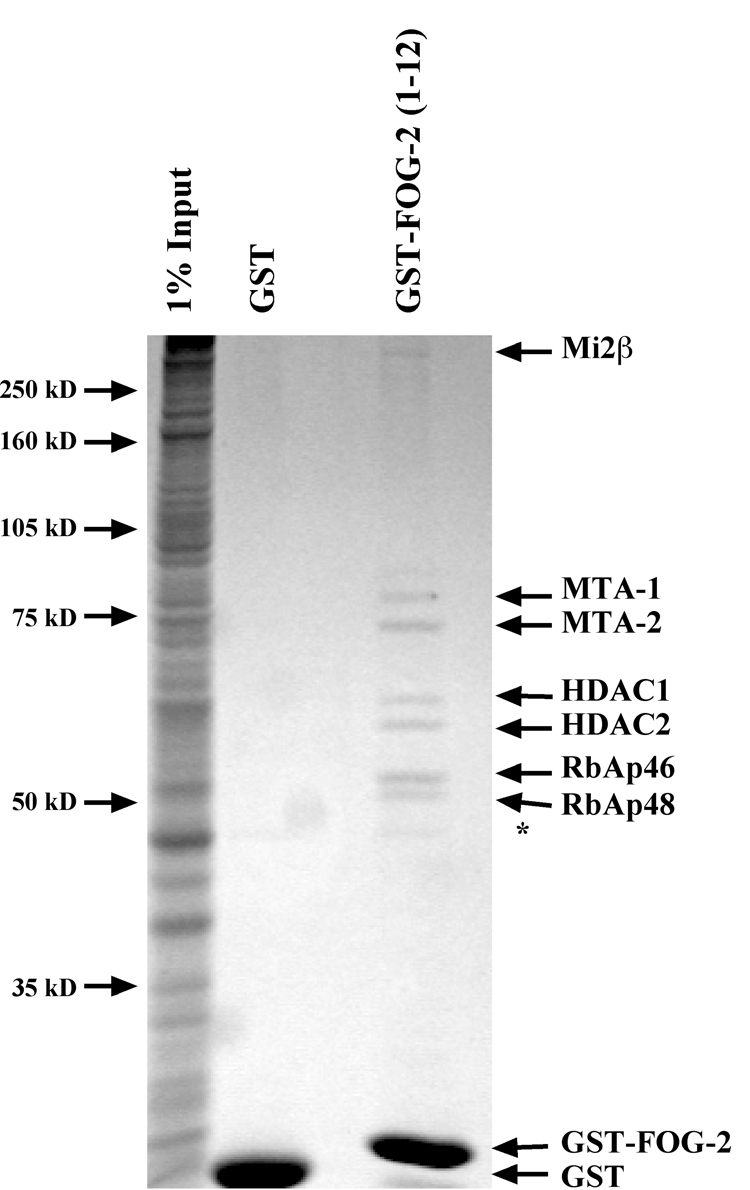
Figure 1. Affinity Purification of FOG-2 Interacting Proteins.
Affinity purified glutathione-S-transferase (GST) or GST fused to the first 12 amino acids of FOG-2 (GST-FOG-2(1–12)) and was incubated with 3.6 milligrams of rat cardiocyte nuclear extract overnight at 4°C. Complexes were purified using glutathione sepharose beads, resolved by SDS-PAGE, and visualized by staining with GelCode reagent. Protein identification was performed by MALDI-TOF mass spectrometry on each visualized subunit. The * indicates a polypeptide isolated using both GST and GST-FOG-2(1–12).