Abstract
This study assessed the behaviour of angiotensin II (Ang II) receptors in an experimental hypothyroidism model in male Wistar rats. Animals were subjected to thyroidectomy and resting for 14 days. The alteration of cardiac mass was evaluated by total heart weight (HW), right ventricle weight (RVW), left ventricle weight (LVW), ratio of HW, RVW and LVW to body weight (BW) and atrial natriuretic factor (ANF) expression. Cardiac and plasma Ang II levels and serum T3 and T4 were determined. The mRNA and protein levels of Ang II receptors were investigated by RT-PCR and Western blotting, respectively. Functional analyses were performed using binding assays. T3 and T4 levels and the haemodynamic parameters confirmed the hypothyroid state. HW/BW, RVW/BW and LVW/BW ratios and the ANF expression were lower than those of control animals. No change was observed in cardiac or plasma Ang II levels. Both AT1/AT2 mRNA and protein levels were increased in the heart of hypothyroid animals due to a significant increase of these receptors in the RV. Experiments performed in cardiomyocytes showed a direct effect promoted by low thyroid hormone levels upon AT1 and AT2 receptors, discarding possible influence of haemodynamic parameters. Functional assays showed that both receptors are able to bind Ang II. Herein, we have identified, for the first time, a close and direct relation of elevated Ang II receptor levels in hypothyroidism. Whether the increase in these receptors in hypothyroidism is an alternative mechanism to compensate the atrophic state of heart or whether it may represent a potential means to the progression of heart failure remains unknown.
It is now clear that the renin–angiotensin system (RAS) acts globally to control blood pressure, and that RAS components act locally within individual organs and under differential regulation (Bader, 2002). The biological actions of RAS are largely related to the effects of the octapeptide angiotensin II (Ang II) and its binding to specific Ang II receptors (Dillmann, 1990; De Gasparo et al. 2000). In addition to these well-known actions, circulating and locally generated Ang II exert other non-haemodynamic effects, stimulating cardiomyocyte growth and fibrosis in adult myocardium, modulating the cardiac hypertrophy process (Morgan & Baker, 1991).
Two pharmacologically distinct subclasses of Ang II receptor, type I (AT1) and type II (AT2), have been identified based on their inhibition by the non-peptide antagonists losartan (AT1) and PD 123319 (AT2) (Chiu et al. 1989). Although both receptors have a seven-transmembrane domain structure typical of G protein-coupled receptors, AT1 and AT2 receptors have different functional properties and signal transduction mechanisms (Ichihara et al. 2001).
While almost all the known physiological effects of Ang II are mediated through the AT1 receptor (Sadoshima & Izumo, 1993), the biological effects associated with the AT2 receptor remain largely unknown. In the heart, Ang II affects cardiac remodelling, contractility and cell growth, most of which can be attributed to activation of the AT1 receptor (Berry et al. 2001; Booz, 2004). In contrast, the growth-inhibitory effects of the AT2 receptor are at least partially mediated by the activation of phosphotyrosine phosphatases that inactivate mitogen-activated protein kinases (MAPK) (Tsuzuki et al. 1996; Horiuchi et al. 1999). However, certain studies have shown that both AT1 and AT2 receptors can act similarly, promoting cardiac hypertrophy, cellular growth and apoptosis (Schelling et al. 1991; Marchant et al. 1993).
Recent data suggest that the tissue RAS may be important in the regulation of local tissue function and can be modulated depending on the specific stimulus, such as hormonal or external signals (Klein, 2003). Some authors reported that the local RAS plays a primary role in the development of cardiac hypertrophy in hyperthyroidism (Kobori et al. 1997). In addition, we recently demonstrated that RAS inhibitors prevent the cardiac hypertrophy induced by thyroid hormone (Hu et al. 2003) and that the thyroid hormone modulates in a tissue specific manner other components of RAS such as angiotensin-converting enzyme (Carneiro-Ramos et al. 2006), providing further evidence for a close relationship between the RAS and thyroid hormones.
Hypothyroidism has been associated with a reduced cardiac performance and consequent decrease in cardiac mass, due to a both diminished gene expression and cytoplasmatic protein levels (Klein, 1988; Sernia et al. 1993). Although the hypothyroidism is a rare pathology, growing evidence suggests a strong link between low thyroid function and worsening outcome in patients with heart disease (Hak et al. 2000; Biondi et al. 2002; Iervasi et al. 2003). Therefore, depending on the severity of hypothyroidism, heart failure (HF) may be incurred and might represent a determining factor directly implicated in the evolution and prognosis of these patients. At this moment, several potential mechanisms by which low thyroid function may contribute to HF have been identified. Hypothyroidism may lead to (1) altered blood lipids and accelerated atherosclerosis, (2) stimulation of myocardial fibrosis, (3) vasoconstriction, and (4) induction of a gene programme resembling that of pathological hypertrophy. Although certain studies have demonstrated that the absence of circulating thyroid hormone correlates to a decrease in angiotensin converting enzyme and renin activities in plasma (Sernia et al. 1993; Kobori et al. 1999), the impact of hypothyroidism upon of local RAS in the heart is still unknown. The aim of the present study was to investigate the effect of hypothyroidism on cardiac Ang II levels and its receptor expression in rats. We have observed an increase on AT1 and AT2 receptors levels in the heart of hypothyroid animals which was not accompanied by Ang II changes. Primary cultures of cardiomyocytes were assessed and confirmed in vivo results, showing a direct effect promoted by low thyroid hormone levels and discarding possible influence of haemodynamic parameters.
Methods
Animals and experimental procedures
All surgical procedures and protocols were performed in accordance with the Ethical Principles in Animal Research, set forth by the Brazilian College of Animal Experimentation, and were approved by the Biomedical Sciences Institute/USP Ethics Committee for Animal Research. Animals were handled according to the National Institutes of Health guidelines. Wistar rats weighing 220–250 g were obtained from the University of São Paulo, Institute of Biomedical Sciences in São Paulo, Brazil. The rats were given free access to standard rat chow and water until the time of the experiment and were housed in a temperature- and light-controlled (24°C; 12 h light–dark cycle) environment. All groups were studied concurrently and at the same age. Rats were randomized into two groups: control and hypothyroid (Hypo). Control animals did not receive any treatment and, in the Hypo group, the rats were surgically thyroidectomized after ketamine (25 mg ml−1) and xylazine (5 mg ml−1) anaesthesia subcutaneously administrated and treated with methimazole (0.05%) plus calcium chloride (4.5 mm) added to their drinking water during 14 days (Giannocco et al. 2004). Thyroidectomy was simulated in the control group, without removal of the thyroid gland.
All rats were killed after 14 days of hypothyroidism by decapitation. The methimazole and calcium chloride were purchased from the Sigma Chemical Company (St Louis, MO, USA).
Hemodynamic parameters
In all rats, indirect systolic blood pressure (SBP) and heart rate (HR) were determined daily at approximately the same time of day by tail-cuff plethysmography (Kent Scientific, Litchfield, CT, USA). In addition, BW was measured daily, and SBP and BW were determined last immediately before kill. Rats were familiarized with the apparatus for a total of 7 days prior to the measurements being taken.
Tissue preparation
Rats were killed by decapitation. Trunk blood was collected without anticoagulant, centrifuged at 3000 r.p.m. for 15 min at 4°C, and then stored at −20°C. Hearts were weighed, right and left ventricle were obtained, and all samples were frozen in liquid nitrogen and stored at −80°C for later analysis of RNA and protein levels.
Determination of cardiac mass and microscopic studies
Cardiac mass was assessed by the measurement of wet right and left ventricle weight (RVW and LVW, respectively). The RVW or LVW to body weight (BW) ratio was calculated in milligrams/grams. The cardiac mass was confirmed by analysing ventricles weight as dry weight (data not shown). Moreover, we analysed atrial natriuretic factor (ANF) mRNA expression by RT-PCR, considering that ANF is a usual cardiac hypertrophy marker (Ladenson et al. 1988; Wong et al. 1989; Eppenberger-Eberhardt et al. 1997). To evaluate the cardiac morphology in both groups, the hearts were transversely sectioned at the midportion between apex and base. A cross-sectional slice was removed and fixed in neutral formalin (10%) at room temperature for 48–72 h. The slice was dehydrated in an increasing alcohol series, immersed in benzene for 20 min, and embedded in paraffin. The samples were sectioned at 5 μm, and 12 non-consecutive sections were collected and stained with haematoxylin and eosin. The images were performed with a magnifying glass of ×4 (MZ6 – Leica).
Serum hormone measurements
Determination of the levels of free triiodothyronine (T3) and free T4, in the serum of control and hypothyroid groups, was performed by a commercial assay using a radioimmunoassay kit (Schering Cis Bio International, France).
Angiotensin II measurements
Hearts were homogenized with 0.045 n HCl in ethanol (10 ml g−1 of tissue) containing p-hydroxymercury benzoate 0.9 μm, 1,10-phenanthroline 131.5 μm, phenylmethylsulphonyl fluoride (PMSF) 0.9 μm, pepstatin A 1.75 μm, EDTA 0.032%, and protease-free bovine serum albumin (BSA) 0.0043% and evaporated. After evaporation, the samples were dissolved in 0.003% trifluoroacetic acid (TFA). Blood samples for Ang II peptide measurements were collected in polypropylene tubes containing p-hydroxymercury benzoate 1 mm, 1,10-phenanthroline 30 mm, PMSF 1 mm, pepstatin A 1 mm, and EDTA 7.5% (50 μl (ml blood)−1). After centrifugation, plasma samples were frozen and stored at −80°C. Peptides were extracted onto a Bond-Elut phenylsilica cartridge (Varian, Palo Alto, CA, USA). The columns were preactivated by sequential washes with 10 ml of 99.9% acetonitrile–0.1% heptafluorobutyric acid (HFBA) and 10 ml of 0.1% HFBA. Sequential washes with 10 ml of 99.9% acetonitrile–0.1% HFBA, 10 ml of 0.1% HFBA, 3 ml of 0.1% HFBA containing 0.1% BSA, 10 ml of 10% HFBA and 3 ml of 0.1% HFBA were used to activate the columns. After sample application, the columns were washed with 20 ml of 0.1% HFBA and 3 ml of 20% HFBA. The adsorbed peptides were eluted with 3 ml of 99.9% acetonitrile–0.1% HFBA into polypropylene tubes rinsed with 0.1% BSA. After evaporation, angiotensin peptide levels were measured by radioimmunoassay (RIA), as previously described. Protein concentration in the crude homogenates was determined by the Bradford method (Bradford, 1976).
Primary cultures of cardiomyocytes
Primary cultures of neonatal rat ventricular cardiac myocytes were prepared by enzymatic disaggregation, as previously described by us (Barreto-Chaves et al. 2000). Briefly, hearts from neonatal (1–3 days old) Wistar rats were excised and ventricles were minced and subjected to multiple enzymatic digestions at 37°C using a mixture of collagenase type 2 (Worthington) and pancreatin (Life Technologies). Four to five subsequent digestions, each lasting a period of 30 min, were performed. The solution obtained in each digest was transferred to a tube containing 1 ml newborn calf serum (NCS; Life Technologies) and centrifuged. Each cell pellet was resuspended in NCS. Dissociated cells were pooled. To segregate myocytes from non-myocytes, this cell suspension was layered onto a discontinuous Percoll (Pharmacia LKB Biotechnology Inc.) density gradient, consisting of two phases. Myocytes were migrated to the interface of the discontinuous layers, and non-myocytes were migrated to the surface of the layer of Percoll. The cells were collected and washed to remove all traces of Percoll. Cellular viability was estimated by the Trypan blue method and after that, the cells were counted and plated. Myocytes were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) containing 5% NCS and 10% horse serum (HS; Life Technologies), supplemented with antibiotics (penicillin, streptomycin; Life Technologies). Cell preparations contained over 95% myocytes, as judged by morphology and spontaneous contraction. After preparation, cells were allowed to adhere to the dishes for 48 h and maintained in DMEM containing 5% NCS and 10% HS. To induce the hypothyroidism in vitro, some cultures were maintained for 24 h in medium containing hypothyroid HS and NCS obtained by charcoal stripped serum method (Cataldo et al. 1982). After that, both control and hypothyroid cultures were maintained in medium containing serum 0.5% for 24 h. Total protein was obtained and the concentrations analysed by the Bradford method (Bradford, 1976). One hundred and fifty micrograms of total protein were resolved by electrophoresis according to Western blot analysis.
RT-PCR analysis
Total RNA from adult rat right and left ventricles or from cardiomyocytes cultures was isolated with Trizol LS reagent (Life Technologies) in accordance with the manufacturer's instructions. The cDNA species were synthesized with SuperScript II (Life Technologies) from 2 μg of total RNA in a total volume of 20 μl with an oligo (dT) primer in accordance with the manufacturer's instructions. cDNA reactions were performed for 1 h at 42°C and stopped by boiling for 5 min. Two microlitres of cDNA was used as a template for PCR with specific primers for angiotensin II receptors. β-Actin-PCR was performed as a control for cDNA synthesis. Two microlitres of the RT reaction mix was used for PCR in a total volume of 25 μl using the concentration of 0.5 μm of each primer indicated below and 50 μm of dNTP and 1 U Taq polymerase (Life Technologies) in the supplied reaction buffer. To find the ideal conditions for the PCR reactions, we initially constructed a curve of the number of cycles performed for each gene studied and another temperature curve and then points that were always localized in the linear phase were chosen.
The PCR cycling conditions were as follows. For the AT1 receptor: 1 min at 94°C, 1.5 min at 59.6°C, 1 min 30 s at 72°C (494 bp amplification product in 32 cycles); for the AT2 receptor: 1 min at 94°C, 1.5 min at 60°C, 1 min 30 s at 72°C (325 bp amplification product in 40 cycles); for ANF: 1 min at 94°C, 1 min at 54°C, 1 min 30 s at 72°C (458 bp amplification product in 16 cycles); and for β-actin: 1 min at 94°C, 1.5 min at 59.8°C, 1 min 30 s at 72°C (210 bp amplification product in 34 cycles). All PCRs were carried out for an initial 3 min denaturation step at 94°C and a final 10 min extension at 72°C. Ten microlitres of the PCR reaction was analysed on a 1.5% agarose gel. The following sets of primers were used: for AT1: 5′-CCC GGG GGC CAC TAG CAC CTC A-3′ and 5′-GCC TGG ACC ACG GGA ACC TT-3′; for AT2: 5′-CCC ATA GCT ATT GGT CTT CAG CAG ATG-3′ and 5′-GCA TGA GTG TTG ATA GGT ACC AAT CGG-3′; for ANF: 5′-GGC TCC TTC TCC ATC ACC AA-3′ and 5′-TGT TAT CTT CGG TAC CG-3′ and for β-actin: 5′-TAT GCC AAC ACA GTG CTG TCT GG-3′ and 5′-TAC TCC TGC TTC CTG ATC CAC AT-3′. Oligonucleotides were obtained from Invitrogen, Brazil. Using an image analysis system, the densitometric intensity related to AT1, AT2, ANF and β-actin bands were converted to numerical values. The mRNA expression was expressed as mRNA of the gene of interest and mRNA β-actin ratio in arbitrary units (densitometric intensity).
Western blot analysis
Proteins from total heart, and right and left ventricles or from cell cultures were obtained using digestion buffer (KCl 90 mm, Hepes 10 mm, MgCl2 3 mm, EDTA 5 mm, glycerol 1%, DTT 1 mm, SDS 0.04%). Protein concentrations were analysed by the Bradford method (Bradford, 1976). One hundred and fifty micrograms of total protein were resolved by electrophoresis on 5% stacking 15% polyacrylamide-SDS gels, and the resolved proteins were transferred to nitrocellulose membrane (Bio-Rad). The membrane was stained with Ponceau solution, to demonstrate that the protein concentration was similar in the different lanes. The membrane was then washed with TBST (Tris 50 mm, NaCl 150 mm, pH 7.5 and Tween-20 2%) for 10 min at room temperature. After this, the membrane was incubated at 37°C for 1 h 30 min with polyclonal antibody against AT1 (1: 1000), AT2 (1: 250) and α-actinin (1: 1000) (SC-1173, SC-7420 and SC-15335, respectively) in TBST. The antibody against AT1 used in the present study recognizes both AT1a and AT1b subtypes. After washing the membrane, the secondary antirabbit IgG antibody conjugated with peroxidase (Amersham Biosciences) at a 1: 1000 dilution in TBST was added for 1 h at room temperature. The membrane was washed again with TBST and was incubated with ECL detection reagents (Amersham Biosciences), which produced a chemiluminescence signal that was detected by exposure to X-ray film. The protein bands were quantified by densitometry and the band density was then calculated.
Binding assays
Sections (9 μm) were serially cut starting from the central area of the right and left ventricle, mounted onto 1% gelatinized slides and dried at 4°C. The slices were preincubated in the assay buffer (1 × PBS plus BSA 0.2%, bacitracin 0.005%, 2.5 × 10−5m phenylmethylsulphonyl fluoride and 6 × 10−4m 1,10-phenanthroline) for 10 min at 4°C. Slices were subsequently incubated with the assay buffer containing fluorescent-labelled Ang II (rhodamine–Ang II) for 1 h at 4°C (total binding). For AT1 receptor binding, 10−5m of losartan was added to the rhodamine–Ang II solution. For AT2 receptor binding 10−5m of PD123319 were added to the Rhodamine-Ang II solution. After incubation, sections were rinsed (three times for 30 s each in assay buffer) succeeded by one quick dip in distilled water. Slices were dried under a stream of air at 22–24°C. All the steps were performed in a dark room. Relative fluorescence measurements were performed on a confocal microscope (Zeiss 510 Meta) using a 40× oil-immersion objective lens.
Statistical analysis
Data are presented as means ±s.e.m. and, in some cases, they are expressed as the percentage of variation in relation to the control group. n represents the number of animals or the number of different cardiomyocytes preparations. Statistical analysis was performed using one-way ANOVA, and P-values < 0.05 were considered statistically significant. Tukey's post hoc test was used to make individual comparisons between groups when a significant change was observed in the ANOVA.
Results
Haemodynamic parameters
Table 1 shows the effect of thyrodectomy on body weight (BW), heart weight (HW), and haemodynamic parameters including systolic blood pressure (SBP) and heart rate (HR). BW at the beginning of the experiment was the same in both groups. However, 14 days after thyroidectomy, the hypothyroid group showed a significant decrease in BW, compared to the control group (241.18 ± 7.03 versus 283.92 ± 6.75 g in control, P < 0.001). Heart weight (HW) was lower in the hypothyroid rats than in control animals (0.79 ± 0.015 versus 0.57 ± 0.007 g in hypothyroid rats, P < 0.001). SBP was also significantly lower in the hypothyroid group (96 ± 2.6 mmHg) than in the control group (129 ± 1.1 mmHg, P < 0.001), as was HR (315 ± 4.7 in hypo versus 392 ± 3.4 beats min−1 in control group, P < 0.001).
Table 1.
Body weight (BW), heart weight (HW) and haemodynamic parameters in control and hypothyroid groups
| Groups | n | Body weight (Day 0) (g) | Body weight (End) (g) | Heart weight | Systolic blood pressure (mmHg) | Heart rate (beats min−1) |
|---|---|---|---|---|---|---|
| Control | 7 | 250.57 ± 4.41 | 283.92 ± 6.75 | 0.79 ± 0.015 | 129 ± 1.1 | 392 ± 3.4 |
| Hypo | 7 | 256.37 ± 5.75 | 241.18 ± 7.03* | 0.57 ± 0.007* | 96 ± 2.6* | 315 ± 4.7* |
Values are expressed as means ±s.e.m.; n, number of rats
P < 0.001 versus control.
Serum concentrations of T3 and T4, as well as cardiac and plasmatic Ang II levels, are summarized in Table 2. Levels of T4 were lower in thyroidectomized rats in relation to control animals (5.37 ± 0.35 versus 47.49 ± 2.6 ng ml−1, respectively, P < 0.001). Serum T3 levels were also significantly lower in hypothyroid rats compared to control animals (0.64 ± 0.02 versus 1.4 ± 0.05 ng ml−1, respectively, P < 0.001). Alterations in serum hormone concentration, associated with the loss in cardiac mass and the decrease in the RH and SBP haemodynamic parameters, served as an index of the hypothyroid state of the animals. The cardiac Ang II levels in the hypothyroid group were not significantly different from the control group. Similarly, plasma angiotensin II levels in thyrodectomized rats were not significantly different from the control group.
Table 2.
Serum thyroid hormone and angiotensin II measurements
| Group | Plasma Ang II (%) | T3 (ηg ml−1) | T4 (ng ml−1) | Cardiac Ang II (%) |
|---|---|---|---|---|
| Control | 1.4 ± 0.05* (6) | 47.49 ± 2.6 (6) | 100 (11) | 100 (10) |
| Hypo | 0.64 ± 0.02* (6) | 5.37 ± 0.35* (6) | 87.27 ± 7.72 (10) | 107.89 ± 8.07 (6) |
Values are expressed as means ±s.e.m.; number of rats shown in parentheses.
P < 0.001 versus control.
Cardiac mass and ANF expression
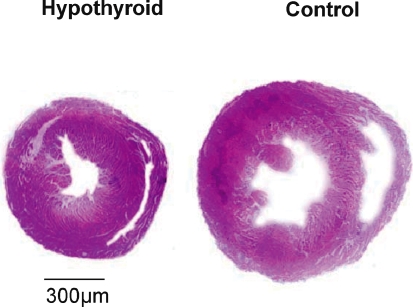
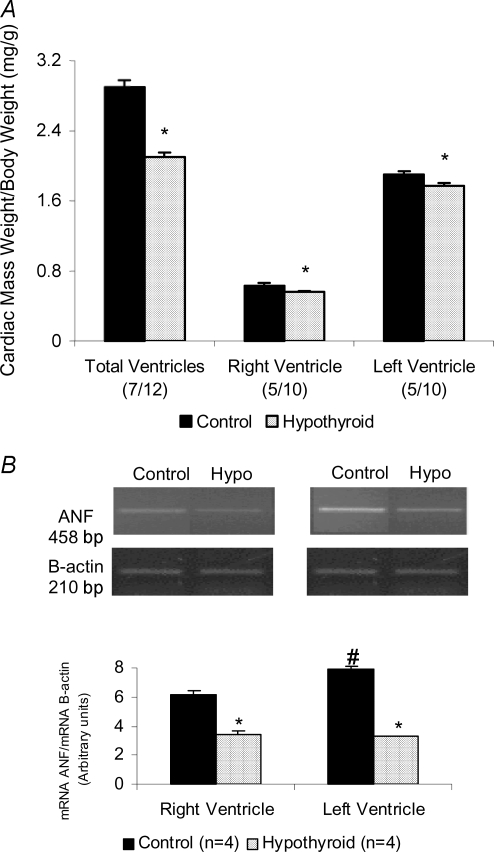
In relation to cardiac morphology, we observed an important atrophy in both right and left ventricles shown in Fig. 1. Heart weight (HW) to body weight (BW) ratio and ANF mRNA levels are presented in Fig. 2. The HW/BW ratio was considerably lower in thyroidectomized rats when compared to the control group (2.1 ± 0.05 versus 2.9 ± 0.08 in control) (Fig. 2A). Besides, the decrease on cardiac mass was observed in both ventricles (12% in RV and 11% in LV). To confirm the cardiac atrophy induced by absence of thyroid hormone levels we also evaluated the ANF gene expression, using semiquantitative RT-PCR (Fig. 2B). A decrease in the ANF mRNA/β-actin mRNA ratio was observed in hearts from thyroidectomized rats. ANF levels in the RV were 55.08% lower than the control animals and 41.51% in LV. These results showed that the privation of thyroid hormone promoted as expected a significant decrease in cardiac muscle mass.
Figure 1.
Hearts transversely sectioned at the midportion between apex and base in control and hypothyroid group Non-consecutive sections of 5 μm were stained with haematoxylin and eosin.
Figure 2.
A, cardiac weight to body weight ratio (in mg/g) in total ventricles, right (RV) and left ventricle (LV) in control and hypothyroid groups. *P < 0.001 versus control. B, ANF mRNA levels evaluated by ANF mRNA/β-actin mRNA ratio in RV and LV in control and hypothyroid groups. *P < 0.01 versus control, #P < 0.001 versus RV control. Values expressed as means ±s.e.m.
Angiotensin II receptors – gene expression, protein levels and functional assays
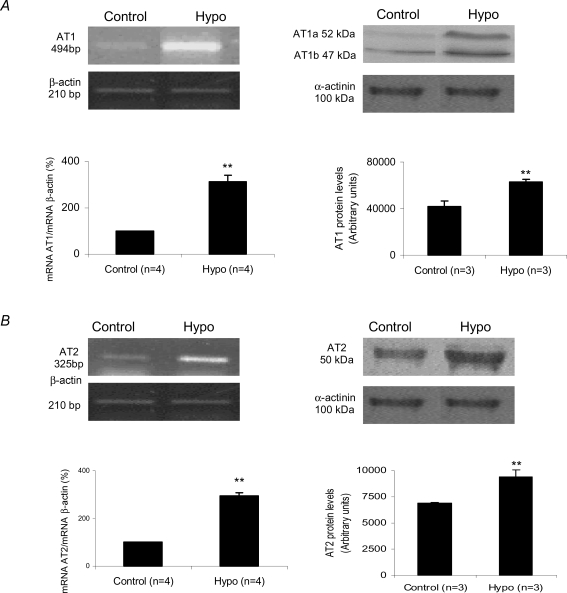
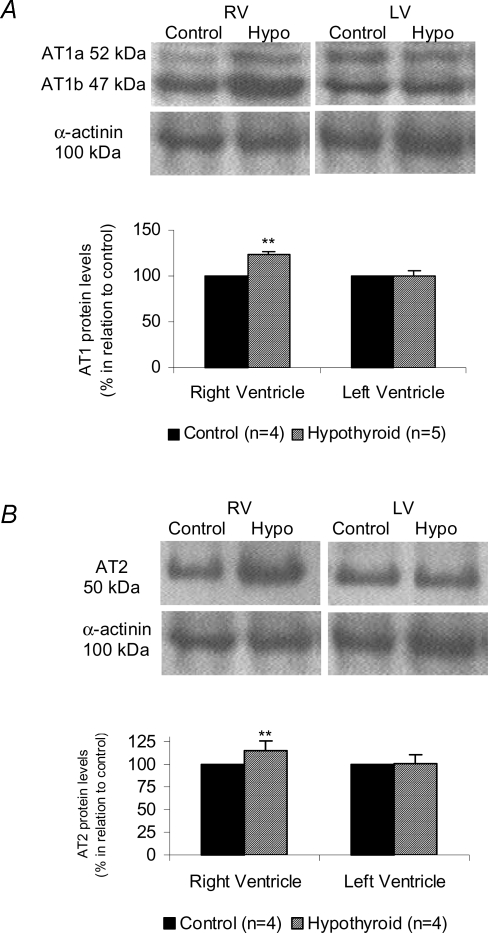
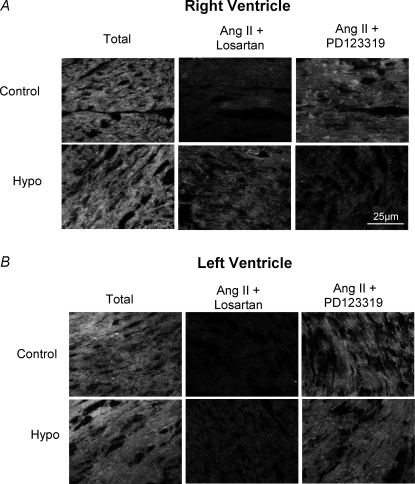
Gene expression and protein levels of cardiac Ang II receptors in both experimental groups are shown in Fig. 3. The AT1 gene expression in the hypothyroid group was significantly increased compared to the control group (313.73 ± 53.71%versus 100% in control) (P < 0.05). The increase in AT1 gene expression in the hearts of thyroidectomized rats was accompanied by an elevation in AT1 protein levels (23.25%), represented by AT1a plus AT1b subtype levels, as demonstrated by Western blotting (Fig. 3A). With regard to the AT2 receptor (Fig. 3B), the Hypo animals also presented a significant increase in mRNA expression (193.94%) and protein levels (37.10%) when compared to control animals (100%). Because the thyroid hormone promotes a different degree of the myocyte hypertrophy in the right and left ventricles (Gerdes et al. 1987), we analysed in the experimental hypothyroidism the Ang II receptors expression separately in both ventricles (Fig. 4). Surprisingly, there was a significant increase on AT1 and AT2 receptors only in right ventricle, we did not observe any change in left ventricle. Considering that the total AT1/AT2 protein content may not have any functional consequences, binding assays were performed in right and left ventricle (Fig. 5). This qualitative method showed that both receptors were able to bind Ang II in right (Fig. 5A) and left (Fig. 5B) ventricles.
Figure 3.
A, angiotensin type 1 receptor mRNA expression given by AT1 mRNA/β-actin mRNA ratio and protein level evaluated by AT1 levels in control and hypothyroid groups. AT1 protein levels refer to AT1a plus AT1b densitometric values. **P < 0.05 versus control. B, angiotensin type 2 receptor mRNA expression given by AT2 mRNA/β-actin mRNA ratio and protein level evaluated by AT2 levels in control and hypothyroid groups. **P < 0.05 versus control. Values expressed as means ±s.e.m.
Figure 4.
A, angiotensin type 1 receptor protein level in right ventricle (RV) and left ventricle (LV) evaluated by AT1 levels in control and hypothyroid groups. AT1 protein levels refers to AT1a plus AT1b densitometric values. **P < 0.05 versus Control. B, angiotensin type 2 receptor protein level in right ventricle (RV) and left ventricle (LV) evaluated by AT2 levels in control and hypothyroid groups. **P < 0.05 versus Control. Values expressed as means ±s.e.m.
Figure 5.
Receptor binding assays used to evaluate the functional state of angiotensin II receptors (AT1 and AT2) in control and hypothyroid groups A, sections (9 μm) from the central area of the right ventricle (RV) showing rhodamine–angiotensin II specific binding total and in the presence of specific inhibitor of AT1 (losartan) or AT2 (PD123319). B, sections (9 μm) from the central area of the left ventricle (LV) showing rhodamine–angiotensin II specific binding total and in the presence of specific inhibitor of AT1 (losartan) or AT2 (PD123319).
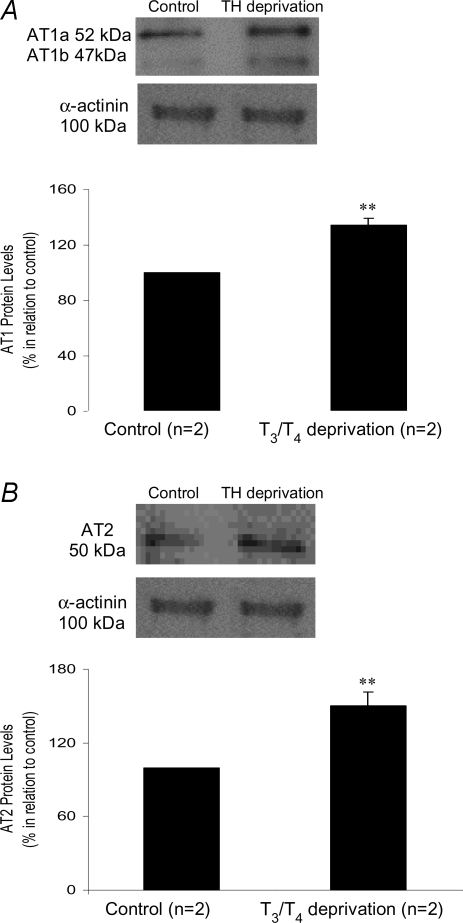
In order to rule out a possible effect of thyroid hormone absence on haemodynamic parameters influencing the results, experiments using primary cultures of cardiomyocytes were performed. AT1 and AT2 receptors expression was significantly increased after the cells had been submitted to T3 and T4 depleted medium for 24 h. In this condition, there was a 131.5 ± 5.02% increase in AT1 expression and 150.4 ± 11.17% in AT2 expression, consistent with the results obtained in vivo after thyroxine deprivation (Fig. 6A and B).
Figure 6.
A, angiotensin type 1 receptor protein level evaluated by AT1 expression levels in control cells and under T3/T4 deprivation. AT1 protein levels refer to AT1a plus AT1b densitometric values. B, angiotensin type 2 receptor protein level evaluated by AT2 expression levels in control cells and under T3/T4 deprivation. **P < 0.05 versus control. Values expressed as mean ±s.e.m.
Discussion
Thyroid disorders are the most common of all endo-crine diseases. In adults, the symptoms may be reversible through adjustment of circulating thyroid hormone levels (Kahaly & Dillmann, 2005). However, the thyroxine replacement therapy remains unsettled since some beneficial and some disadvantageous consequences have been identified (McDermott et al. 2001; Meier et al. 2001). The effects of hypothyroidism on the cardiovascular system include changes in cardiac physiology such as decreased cardiac output and contractility, increased systemic vascular resistance and decreased heart rate (Biondi et al. 2002; Ohga et al. 2002; Pantos et al. 2003). In contrast to hyperthyroidism, the contribution of local RAS to function and structure maintenance for normal cardiac activity, in the face of low levels of thyroid hormone, has not been studied yet. Then, in this work we observed that in the hypothyroid cardiac state, the plasma Ang II levels were unaffected whereas both Ang II receptor subtypes, AT1 and AT2, were up-regulated at the mRNA and protein levels. This effect was independent of simple changes in systemic actions, since increased levels of Ang II receptor expression were also observed in TH deprived cardiomyocytes.
Thyroid hormones appear to be one of the important endocrine factors involved in the maintenance of basal plasma angiotensinogen levels, since hyperthyroidism increases and hypothyroidism decreases angiotensinogen production, as observed in rats (Bouhnik et al. 1981; Dzau & Herrmann, 1982). Moreover, Marchant et al. (1993) showed that hypothyroid rats presented a significant decrease in plasma renin activity, but not in plasma renin concentration, establishing a positive correlation between thyroid hormone levels and the renin–angiotensin system.
The role of RAS in the development of the cardiac hypertrophy induced by thyroid hormone has been previously studied by us and other authors (Kuzmits et al. 1985; Kobori et al. 1999; Hu et al. 2003; Carneiro-Ramos et al. 2006). However, the behaviour of RAS in a T4 privation condition is not completely understood yet. As previously described, although certain studies have presented a close relationship between the thyroid hormone levels and the activation of RAS, in this study we demonstrated, for the first time, that in the condition of hypothyroidism, the Ang II receptors are markedly increased in the heart due to an important increased expression in right ventricle. Hypothyroidism was proven by decreased serum thyroid hormone levels, haemodynamic parameters and by ANF gene expression analysis. These data are in agreement with previous observations, in hypothyroidism, of a reduced gene transcription and translation with consequent loss of cardiac mass and contractile machinery (Kahaly & Dillmann, 2005). In addition, results confirm that basal levels of thyroid hormone are very important for cardiac tissue development and for maintenance of haemodynamic parameters.
Regarding Ang II levels and receptors expression after thyroidectomy, there are important issues to highlight. Firstly, both plasma and cardiac Ang II levels did not change after thyroidectomy. Thus, hypothyroidism did not alter the Ang II concentration compared to that of control animals. Furthermore, data obtained in our laboratory show that surgically thyroidectomized rats that were treated with methimazole for 14 days presented a significant decrease in serum and pulmonary angiotensin converting enzyme (ACE) activity compared with the control group. On the other hand, the cardiac ACE activity was significantly increased in hypothyroid animals (data not shown). Thus, it is clear that local Ang II generation depends on ACE, in part, since other enzymes are able to convert angiotensin I into angiotensin II, such as chymases and elastase-2 (Balcells et al. 1997; Santos et al. 2002). Secondly, cardiac AT1 and AT2 receptors showed a significant increase in mRNA and protein levels in thyroidectomized rats. Moreover, the animals that had been thyroidectomized for 14 days and that were supplemented with T4 at 0.100 mg (kg BW)−1 for 14 days subsequently presented a normal HW/BW ratio (2.98 ± 0.26, n = 5) and AT1 (98.12 ± 5.15, n = 4) and AT2 (109.26 ± 8.15, n = 4) receptor levels returned to control levels. These data and those obtained in cardiomyocytes cultures after TH privation show that the changes observed in hypothyroid hearts reflect a loss of a direct effect of T3/T4 on the cardiac cells and not an indirect consequence of change in haemodynamic parameters. Therefore, although we did not find any changes in cardiac Ang II concentrations during the hypothyroid state, we clearly observed that the final components of this system – Ang II receptors – are augmented and under thyroid hormone level regulation.
It is well known that Ang II exerts other non-haemodynamic effects, which have been implicated in cardiac cell proliferation, including cardiomyocyte hypertrophy (Aceto & Baker, 1990) and fibroblast hyperplasia (Schelling et al. 1991). Considering the trophic actions of Ang II, one might predict that thyroid hormone deprivation would increase AT1 and AT2 receptor expression in order to compensate the response to limited amounts of thyroid hormone. In fact, in hypothyroidism many key enzymes involved in intracellular Ca2+ regulation and thus related to heart contractility and to trophism of cardiac muscle are increased as a compensatory mechanism. Recently, van Rooij et al. (2007) showed elegantly that a cardiac-specific microRNA (miR-208), encoded by an intron of the α-MHC gene, is required for cardiomyocyte hypertrophy, fibrosis and expression of β-MHC in response to hypothyroidism. Thus, the α-MHC gene, in addition to encoding a major cardiac contractile protein, regulates cardiac growth and gene expression in response to hormonal signalling via miR-208. Furthermore, it has been recently shown that protein kinase C (PKC) increases thyroid hormone receptor expression in heart (Kenessey et al. 2006). Given that PKC is activated by the angiotensin type 1 receptor after Ang II binding, we postulate that Ang II, available in cardiac tissue, can bind to its receptors, activating PKC and, thus, this kinase could be responsible for the elevation in thyroid hormone receptor transcription. Taking together, we hypothesize that the increase in Ang II receptors in hypothyroidism is an alternative mechanism to increase the thyroid hormone sensitivity in cardiac tissue, since this hormone can exert a trophic action on cardiac tissue to compensate the atrophic state of the hypothyroid heart. On the other hand, recently Tang et al. (2005) showed in rats that low thyroid function alone has the potential to cause heart failure, in part due to promoting heart dilatation as consequence of elongation of contracting muscle cells, a specific change typical of heart failure. In this case, the increased Ang II receptor levels may adversely represent a potential and direct means of influencing Ang II-induced changes in the growth and structure of the myocardium and thereby its contribution to the progression of heart failure.
Although the functional significance of a differential distribution of Ang II receptors in right ventricle in comparison to left ventricle requires further investigation, some hypothesis may be elicited. The hypothyroidism is associated with RV systolic and diastolic dysfunction (Turhan et al. 2006) and these disorders may be associated with changes in the proportion and distribution of Ang II receptors in cardiac tissue (Rogg et al. 1996). In contrast, in the hyperthyroidism, right ventricular pressure is increased by a much greater percentage than systemic blood pressure (Alpert et al. 1983), which may result in pulmonary hypertension. In addition, it should be also appreciated that there is a differential distribution of thyroid hormone receptors (TRs) in the heart and the differences in response to T3 action between both ventricles or cardiac conduction system could be related to the regional expression of both TR isoforms (Stoykov et al. 2006). Then, TRα1 and TRα2 are the predominant TRs expressed in the heart and the TH withdrawal results in a decrease in TRα1 and an increase in TRα2. This TRα2 isoform does not bind TH and acts predominantly to suppress expression of genes containing TH response elements (TREs) by forming heterodimers with the TH-binding TR isoforms (Lazar, 1990). Therefore, in heart, TH withdrawal results in lower TRα2, which would possibly enhance the action of TH when levels are low (Sadow et al. 2003).
In the present study, using in vivo and in vitro approaches we have identified, for the first time, a close and direct relation of elevated Ang II receptors levels in the absence of thyroid hormones. Does this also occur in humans? If so, what is the clinical impact on progression of heart disease in hypothyroidism? Clearly, additional studies are needed to better understand the consequences of low thyroid function in heart and the role of renin–angiotensin system under these conditions.
Acknowledgments
We thank Joelcimar Martins da Silva for providing technical assistance and Dr Claudimara F. Lotfi for providing the secondary antibody. This study received financial support in the form of grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo; grants 01/11678-8 and 03/04638-8) and Conselho Nacional de Pesquisa (CNPq).
References
- Aceto JF, Baker KM. [Sar1]angiotensin II receptor-mediated stimulation of protein synthesis in chick heart cells. Am J Physiol Heart Circ Physiol. 1990;258:H806–H813. doi: 10.1152/ajpheart.1990.258.3.H806. [DOI] [PubMed] [Google Scholar]
- Alpert MA, Goldberg SH, Singsen BH, Durham JB, Sharp GC, Ahmad M, Madigan NP, Hurst DP, Sullivan WD. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;68:1182–1193. doi: 10.1161/01.cir.68.6.1182. [DOI] [PubMed] [Google Scholar]
- Bader M. Role of the local renin-angiotensin system in cardiac damage: a minireview focussing on transgenic animal models. J Mol Cell Cardiol. 2002;34:1455–1462. doi: 10.1006/jmcc.2002.2077. [DOI] [PubMed] [Google Scholar]
- Balcells E, Meng QC, Johnson WH, Jr, Oparil S, Dell'Italia LJ. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol Heart Circ Physiol. 1997;273:H1769–H1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- Barreto-Chaves ML, Heimann A, Krieger JE. Stimulatory effect of dexamethasone on angiotensin-converting enzyme in neonatal rat cardiac myocytes. Braz J Med Biol Res. 2000;33:661–664. doi: 10.1590/s0100-879x2000000600007. [DOI] [PubMed] [Google Scholar]
- Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–H2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968–974. doi: 10.1210/jcem.87.3.8302. [DOI] [PubMed] [Google Scholar]
- Booz GW. Cardiac angiotensin AT2 receptor: what exactly does it do? Hypertension. 2004;43:1162–1163. doi: 10.1161/01.HYP.0000128531.39964.c0. [DOI] [PubMed] [Google Scholar]
- Bouhnik J, Clauser E, Strosberg D, Frenoy JP, Menard J, Corvol P. Rat angiotensinogen and des (angiotensin I) angiotensinogen: purification, characterization, and partial sequencing. Biochemistry. 1981;20:7010–7015. doi: 10.1021/bi00527a036. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carneiro-Ramos MS, Silva VB, Santos RA, Barreto-Chaves ML. Tissue-specific modulation of angiotensin-converting enzyme (ACE) in hyperthyroidism. Peptides. 2006;27:2942–2949. doi: 10.1016/j.peptides.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Cataldo NA, Cooper DS, Chin WW, Maloof F, Ridgway EC. The effect of thyroid hormones on prolactin secretion by cultured bovine pituitary cells. Metabolism. 1982;31:589–594. doi: 10.1016/0026-0495(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, et al. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;165:196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Dillmann WH. Biochemical basis of thyroid hormone action in the heart. Am J Med. 1990;88:626–630. doi: 10.1016/0002-9343(90)90530-q. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Herrmann HC. Hormonal control of angiotensinogen production. Life Sci. 1982;30:577–584. doi: 10.1016/0024-3205(82)90272-7. [DOI] [PubMed] [Google Scholar]
- Eppenberger-Eberhardt M, Aigner S, Donath MY, Kurer V, Walther P, Zuppinger C, Schaub MC, Eppenberger HM. IGF-I and bFGF differentially influence atrial natriuretic factor and α-smooth muscle actin expression in cultured atrial compared to ventricular adult rat cardiomyocytes. J Mol Cell Cardiol. 1997;29:2027–2039. doi: 10.1006/jmcc.1997.0408. [DOI] [PubMed] [Google Scholar]
- Gerdes AM, Moore JA, Hines JM. Regional changes in myocyte size and number in propranolol-treated hyperthyroid rats. Lab Invest. 1987;57:708–713. [PubMed] [Google Scholar]
- Giannocco G, DosSantos RA, Nunes MT. Thyroid hormone stimulates myoglobin gene expression in rat cardiac muscle. Mol Cell Endocrinol. 2004;226:19–26. doi: 10.1016/j.mce.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Hu LW, Benvenuti LA, Liberti EA, Carneiro-Ramos MS, Barreto-Chaves ML. Thyroxine-induced cardiac hypertrophy: influence of adrenergic nervous system versus renin-angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1473–R1480. doi: 10.1152/ajpregu.00269.2003. [DOI] [PubMed] [Google Scholar]
- Ichihara S, Senbonmatsu T, Price E, Jr, Ichiki T, Gaffney FA, Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–713. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- Kenessey A, Sullivan EA, Ojamaa K. Nuclear localization of protein kinase C-α induces thyroid hormone receptor-α1 expression in the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2006;290:H381–H389. doi: 10.1152/ajpheart.00576.2005. [DOI] [PubMed] [Google Scholar]
- Klein I. Thyroxine-induced cardiac hypertrophy: time course of development and inhibition by propranolol. Endocrinology. 1988;123:203–210. doi: 10.1210/endo-123-1-203. [DOI] [PubMed] [Google Scholar]
- Klein I. Thyroid hormone and cardiac contractility. Am J Cardiol. 2003;91:1331–1332. doi: 10.1016/s0002-9149(03)00433-8. [DOI] [PubMed] [Google Scholar]
- Kobori H, Ichihara A, Miyashita Y, Hayashi M, Saruta T. Local renin-angiotensin system contributes to hyperthyroidism-induced cardiac hypertrophy. J Endocrinol. 1999;160:43–47. doi: 10.1677/joe.0.1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Ichihara A, Suzuki H, Miyashita Y, Hayashi M, Saruta T. Thyroid hormone stimulates renin synthesis in rats without involving the sympathetic nervous system. Am J Physiol Endocrinol Metab. 1997;272:E227–E232. doi: 10.1152/ajpendo.1997.272.2.E227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmits R, Schwarz M, Weissel M. Effects of variations in thyroid hormone serum concentrations on serum ACE-activity. Horm Metab Res. 1985;17:528–531. doi: 10.1055/s-2007-1013595. [DOI] [PubMed] [Google Scholar]
- Ladenson PW, Bloch KD, Seidman JG. Modulation of atrial natriuretic factor by thyroid hormone: messenger ribonucleic acid and peptide levels in hypothyroid, euthyroid, and hyperthyroid rat atria and ventricles. Endocrinology. 1988;123:652–657. doi: 10.1210/endo-123-1-652. [DOI] [PubMed] [Google Scholar]
- Lazar MA. Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem. 1990;265:17474–17477. [PubMed] [Google Scholar]
- Marchant C, Brown L, Sernia C. Renin-angiotensin system in thyroid dysfunction in rats. J Cardiovasc Pharmacol. 1993;22:449–455. doi: 10.1097/00005344-199309000-00016. [DOI] [PubMed] [Google Scholar]
- McDermott MT, Haugen BR, Lezotte DC, Seggelke S, Ridgway EC. Management practices among primary care physicians and thyroid specialists in the care of hypothyroid patients. Thyroid. 2001;11:757–764. doi: 10.1089/10507250152484592. [DOI] [PubMed] [Google Scholar]
- Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Muller B. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study) J Clin Endocrinol Metab. 2001;86:4860–4866. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- Morgan HE, Baker KM. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991;83:13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Ohga Y, Sakata S, Takenaka C, Abe T, Tsuji T, Taniguchi S, Takaki M. Cardiac dysfunction in terms of left ventricular mechanical work and energetics in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2002;283:H631–H641. doi: 10.1152/ajpheart.00046.2002. [DOI] [PubMed] [Google Scholar]
- Pantos C, Malliopoulou V, Mourouzis I, Sfakianoudis K, Tzeis S, Doumba P, Xinaris C, Cokkinos AD, Carageorgiou H, Varonos DD, Cokkinos DV. Propylthiouracil-induced hypothyroidism is associated with increased tolerance of the isolated rat heart to ischaemia-reperfusion. J Endocrinol. 2003;178:427–435. doi: 10.1677/joe.0.1780427. [DOI] [PubMed] [Google Scholar]
- Rogg H, de Gasparo M, Graedel E, Stulz P, Burkart F, Eberhard M, Erne P. Angiotensin II-receptor subtypes in human atria and evidence for alterations in patients with cardiac dysfunction. European Heart J. 1996;17:1112–1120. doi: 10.1093/oxfordjournals.eurheartj.a015008. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Molecular characterization of angiotensin II induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Sadow PM, Chassande O, Koo EK, Gauthier K, Samarut J, Xu J, O'Malley BW, Weiss RE. Regulation of expression of thyroid hormone receptor isoforms and coactivators in liver and heart by thyroid hormone. Mol Cell Endocrinol. 2003;203:65–75. doi: 10.1016/s0303-7207(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Santos CF, Oliveira EB, Salgado MC, Greene AS. Molecular cloning and sequencing of the cDNA for rat mesenteric arterial bed elastase-2, an angiotensin II-forming enzyme. J Cardiovasc Pharmacol. 2002;39:628–635. doi: 10.1097/00005344-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Schelling P, Fischer H, Ganten D. Angiotensin and cell growth: a link to cardiovascular hypertrophy? J Hypertens. 1991;9:3–15. [PubMed] [Google Scholar]
- Sernia C, Marchant C, Brown L, Hoey A. Cardiac angiotensin receptors in experimental hyperthyroidism in dogs. Cardiovasc Res. 1993;27:423–428. doi: 10.1093/cvr/27.3.423. [DOI] [PubMed] [Google Scholar]
- Stoykov I, Zandieh-Doulabi B, Moorman AF, Christoffels V, Wiersinga WM, Bakker O. Expression pattern and ontogenesis of thyroid hormone receptor isoforms in the mouse heart. J Endocrinol. 2006;189:231–245. doi: 10.1677/joe.1.06282. [DOI] [PubMed] [Google Scholar]
- Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–3130. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Matoba T, Eguchi S, Inagami T. Angiotensin II type 2 receptor inhibits cell proliferation and activates tyrosine phosphatase. Hypertension. 1996;28:916–918. doi: 10.1161/01.hyp.28.5.916. [DOI] [PubMed] [Google Scholar]
- Turhan S, Tulunay C, Ozduman Cin M, Gursoy A, Kilickap M, Dincer I, Candemir B, Gullu S, Erol C. Effects of thyroxine therapy on right ventricular systolic and diastolic function in patients with subclinical hypothyroidism: a study by pulsed wave tissue Doppler imaging. J Clin Endocrinol Metab. 2006;91:3490–3493. doi: 10.1210/jc.2006-0810. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- Wong NLD, Huang, et al. Effects of thyroid status of atrial natriuretic peptide release from isolated rat atria. Am J Physiology. 1989;256:64–67. doi: 10.1152/ajpendo.1989.256.1.E64. [DOI] [PubMed] [Google Scholar]