Abstract
Hypothermia is a significant contributor to mortality in severely injured patients. Rewarming is an enormous challenge, especially in those who require operative or angiographic intervention. In this patient population, external warming methods are only capable of reducing further heat loss, whereas active rewarming adds heat to the body's core but is invasive. This article analyzes our initial experience with a minimally invasive, continuous, automated, and easily portable intravascular rewarming technique using the Alsius Corporation's CoolGard system. The records of 11 hypothermic critically injured patients presenting to our level 1 trauma center over a 6-month period were reviewed. The patients' mean age was 39 ± 22 years, 7 (64%) were male, and 7 (64%) had blunt mechanisms of injury. The mean injury severity score was 40 ± 16, and the mean initial systolic blood pressure was 91 ± 60 mm Hg. The mean core temperature at the initiation of rewarming was 33.6 ± 1.0°C, and the mean rewarming rate was 1.5 ± 1.0°C/h. Six patients died (55%), two of acute exsanguination and four of unsurvivable traumatic brain injuries. One patient developed a deep vein thrombosis at the femoral catheter site and experienced a nonfatal pulmonary embolus. Our experience demonstrates that active intravascular balloon-catheter rewarming represents a practical, automated technique for the immediate and continuous treatment of hypothermia in all phases of the acute care of trauma patients.
The primary objective in the treatment of critically injured patients is control of hemorrhage. The secondary goal is global restoration of normal physiologic metabolism, which includes, but is not limited to, organ structural and functional integrity, contamination control, and normothermia. The Advanced Trauma Life Support (ATLS) framework classifies core temperature hypothermia as mild (32–35°C), moderate (30–32°C), or severe (<30°C) (1). Primary hypothermia is caused by environmental exposure to cold, whereas secondary hypothermia results from inadequate physiologic heat production. In contrast to severe environmental hypothermia, which is associated with a mortality rate of <25%, even moderate hypothermia associated with severe traumatic injury is associated with a nearly 100% mortality risk (2, 3).
Warm blood volume loss, environmental exposure, and significantly reduced metabolic heat production in the hemodynamic shock state of the multi-injured patient all contribute to hypothermia, which in turn leads to exacerbation of coagulopathy and further blood and heat loss. Several reports of consecutive patients have revealed an incidence of initial hypothermia by the above ATLS definitions of approximately 40% and a strong correlation between degree of hypothermia and injury severity (3–5).
Temperatures in the emergency department (ED) and operating room (OR) are typically 21 to 22°C (70–72°F), which puts patients at significant risk of further heat loss. The OR can be warmed to 29°C (85°F); while that temperature can help reduce the rate of heat loss, it is still far below normal body temperature and thus cannot actively warm the patient. Evidence shows that nearly 50% of trauma patients leave the OR hypothermic (5).
Several previous studies have demonstrated the multifactorial benefits of rapid rewarming of critically injured patients, the most effective of which is extracorporeal blood rewarming (6–9). The recent limited availability of disposable extracorporeal rewarming circuits caused us to seek an alternative rewarming device. Alsius Corporation's (Irvine, CA) intravascular balloon-catheter system has been approved in the United States for therapeutic human core cooling and rewarming during or following cardiac or neurologic surgery and following cerebral infarction or intracerebral hemorrhage and was thus employed for core rewarming after traumatic injury (10). This article describes our initial experience with intravascular rewarming using this closed-circuit, thermostat-controlled, warm-water circulating balloon catheter.
METHODS
The records of 12 patients treated with this system by our acute care surgery group at Baylor University Medical Center in Dallas, Texas, from May to October 2007 were retrospectively reviewed. Institutional review board approval was obtained for the study. In addition to temperature and warming data, information was collected regarding demographics, injury patterns and severity, laboratory results, transfusion requirements, hospital course, and outcomes. Eleven critically injured patients, all requiring central venous access for resuscitation, were selected for intravascular re warming based on the attending trauma surgeon's clinical judgment. One additional patient with severe primary hypothermia was treated by the same technique and is described but not included in data analysis.
Automated target core temperature was set at 37°C (98.6°F), and maximum flow rate was used for all 12 patients. The management of the patients and their injuries was not otherwise altered from the treating physicians' usual practice. Standard warming measures, including warm blankets, convective heated-air blankets, and intravenous fluid and blood product in-line warming machines, were initiated immediately and continued throughout the patients' hospital course per usual routine. Internal cavitary lavage was not performed except for the customary use of warm saline irrigation during surgery. Core temperature was monitored using a bladder catheter with a built-in temperature probe.
The outcome under investigation was efficacy of the technique in this patient population as evidenced by warming rate. Additional findings regarding complications and relationships (by Pearson correlation) between time to initiation of rewarming, starting rewarming temperature, rewarming rate, injury severity score (ISS), transfusion requirements, degree of acidosis, degree of coagulopathy, and survival are also discussed.
Equipment
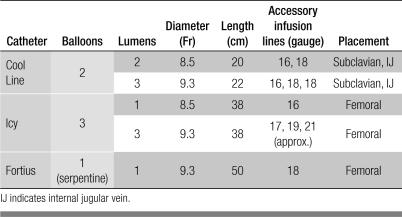
The CoolGard 3000 system (Alsius Corporation, Irvine, CA) (Figure 1) was designed for induction and maintenance of hypothermia after myocardial infarction with cardiac arrest or central nervous system insult. The same system is used for rewarming after the period of therapeutic hypothermia and was therefore employed for intravascular rewarming of hypothermic trauma patients. The machine acts as a thermostat for core body temperature control, with a user-selected target temperature (31–38°C) and warming or cooling rate (0.1°C/h to maximum). Sterile saline from a standard 500-mL hanging bag is actively pumped through the machine and the intravascular catheter balloons in a closed loop at 200 to 240 mL/min, depending on the catheter type. Within the machine, the saline passes first through an air trap and then a metal heat exchanger coil submerged in a temperature-controlled coolant well containing a mixture of propylene glycol and distilled water. The saline then circulates through balloons on the intravascular surface of one of the specially designed central venous catheters at a temperature of 0 to 42°C to deliver or remove heat from the bloodstream (Figure 2). Core temperature is monitored by the machine via a standard YSI-400 compatible bladder, rectal, or esophageal thermometer probe (10).
Figure 1.
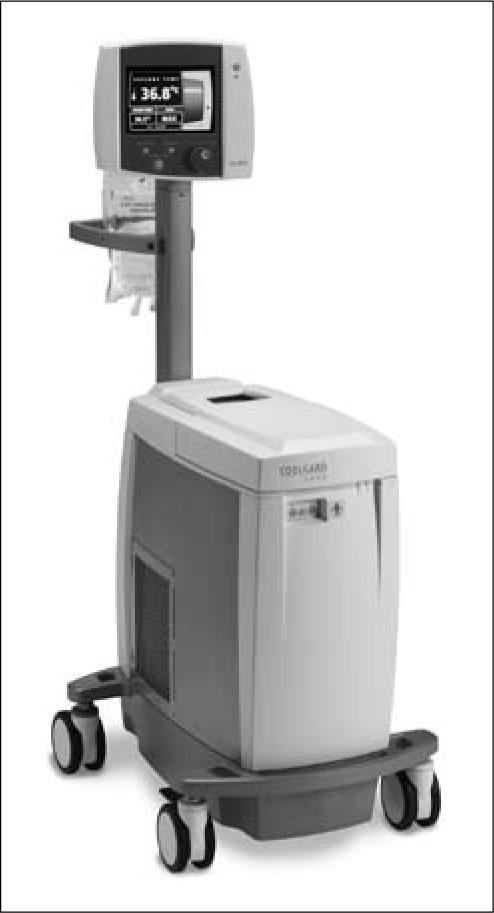
The CoolGard thermal regulation system, which remotely senses changes in a patient's core temperature and automatically adjusts the temperature to the target by use of a catheter with circulating saline. Reprinted courtesy of Alsius.
Figure 2.
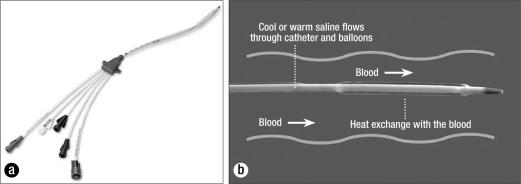
(a) An Alsius catheter. (b) The catheter is inserted into the common femoral vein and resides in the inferior vena cava. Saline flow within the balloons creates a proprietary vortex flow pattern, which maximizes heat exchange with blood as it passes by. Reprinted courtesy of Alsius.
Currently, three catheter types are available for use: Cool Line, Icy, and Fortius (Alsius Corporation). Their characteristics are given in Table 1, including the number and size of medication infusion lumens on each catheter. A 3-lumen Icy catheter placed in the femoral vein was used in the patients in this report.
Table 1.
Catheters used with the CoolGard 3000 system
RESULTS
During the period of this review, 11 hypothermic trauma patients were treated with the intravascular rewarming device. The mean patient age was 39 years, the mean ISS was 40, and the mean revised trauma score was 6.5, indicating a group of significantly injured patients (Table 2). Seven patients (64%) were in shock (systolic blood pressure <90 mm Hg) upon arrival to the ED. Three of the four patients with systolic blood pressure >90 mm Hg were combative and subsequently became hypotensive. Although the patients' mean ED arrival temperature was 35°C, they continued to lose heat at an average rate of 0.6°C/h and had a mean temperature at the start of intravascular rewarming of 33.6°C, despite aggressive implementation of standard hypothermia precautions and warming measures.
Table 2.
Characteristics of 11 trauma patients treated with the CoolGard rewarming system at Baylor University Medical Center
| Variable | Mean ± SD (range) or quantity |
| Age (years) | 39.3 ± 21.6 (16–84) |
| Male:female | 7:4 |
| Blunt:penetrating | 7:4 |
| Taken to the operating room | 5 |
| Taken to the angiography suite | 2 |
| Taken to the intensive care unit | 4 |
| Mean injury severity score | 39.6 ± 15.7 (12–75) |
| Mean revised trauma score | 6.5 ± 4.5 (0–12) |
| Mean arrival systolic blood pressure (mm Hg) | 91.1 ± 60.2 (0–210) |
| Mean arrival temperature (°C) | 35.2 ± 1.1 (33.3.–36.8) |
| Mean rewarming start temperature (°C) | 33.6 ± 1.0 (31.8–35.3) |
| Mean arrival pH | 7.05 ± 0.21 (6.69–7.33) |
| Mean arrival base deficit | 9.0 ± 6.3 (1–18) |
| Mean arrival prothrombin time | 23.0 ± 12.2 (11.5–50.8) |
| Mean units all blood products | 24.5 ± 21.0 (0–73) |
Earlier in the period of review, intravascular warming was not initiated until the patient arrived in the intensive care unit (ICU). However, with experience, the efficacy and feasibility of instituting rewarming earlier with this mobile technique was realized, and emphasis was given to starting rewarming as early as possible, either in the ED or upon arrival to the OR. Seven patients in this report went from the ED directly to either the OR or to the interventional radiology angiography suite to control internal bleeding, with an additional risk of heat loss in these relatively cold environments; three of these patients died. Four patients went directly from the ED to the ICU, three of whom died of unsurvivable traumatic brain injury. Of the five survivors, four had rewarming initiated early in the course of treatment, either in the ED or OR. Only one fatality, due to nonsurvivable brain injury, occurred in a patient whose rewarming began prior to ICU arrival. Of note, the two patients with the lowest ED arrival temperatures, both with extensive penetrating injuries, had intravascular rewarming begun immediately and survived.
As shown in Table 3, the mean warming rate of all 11 patients was 1.5°C/h (SD, 1.0; range, 0.67–4.00), which correlated strongly with the degree of hypothermia (r = 0.67). That is, the more hypothermic an individual patient was at the start of intravascular rewarming, the faster the warming rate. The degree of hypothermia also showed significant correlation to acidosis (for pH r = –0.77), base deficit (r = 0.48), and coagulation factor deficit (for temperature-corrected prothrombin time, r = 0.53), which are also known to negatively influence survival in trauma patients. Starting temperatures and warming rates did not correlate well with ISS, total transfusion amounts, or survival (all r < 0.30) in our small sample.
Table 3.
Rewarming of 11 trauma patients treated with the CoolGard rewarming system at Baylor University Medical Center
| Patient | ED temp (°C) | Procedure | Start of rewarming | Delay interval (min) | Start temp (°C) | Warming rate (°C/h) |
| 1 | 36.1 | IR | ICU | 577 | 33.3 | 1.24 |
| 2 | 36.1 | OR | ICU | 210 | 34.4 | 1.37 |
| 3 | 35.1 | OR | ICU | 180 | 33.5 | 1.09 |
| 4 | 35.3 | OR | OR | 49 | 32.2 | 1.93 |
| 5 | 34.8 | None | ICU | 114 | 33.1 | 0.76 |
| 6 | 33.3 | OR | OR | 79 | 33.6 | 0.77 |
| 7 | 33.7 | None | ED | 85 | 34.7 | 0.67 |
| 8 | 36.8 | None | ICU | 88 | 31.8 | 4.00 |
| 9 | 34.9 | IR | ICU | 160 | 33.1 | 2.74 |
| 10 | 34.3 | None | ED | 130 | 34.3 | 1.08 |
| 11 | 36.5 | OR | OR | 119 | 35.3 | 1.11 |
| Mean | 35.2 | 163 | 33.6 | 1.53 |
ED indicates emergency department; IR, interventional radiology angiography suite; OR, operating room; ICU, intensive care unit.
Table 3 also demonstrates the progressive heat loss over time as a result of therapeutic interventions. Overall, patients lost an average of 1.6°C prior to initiation of rewarming, but the subset whose rewarming was delayed until arrival to the ICU lost an average of 2.4°C. Within this subset, a mean delay of 282 minutes was incurred by surgery or angiography, which represents over 4.5 hours of ongoing net heat loss despite maximal external warming efforts, and lost rewarming time during the acute injury period. For the entire 11-patient group, the delay interval between ED arrival and start of rewarming correlated strongly with mortality (r = 0.66).
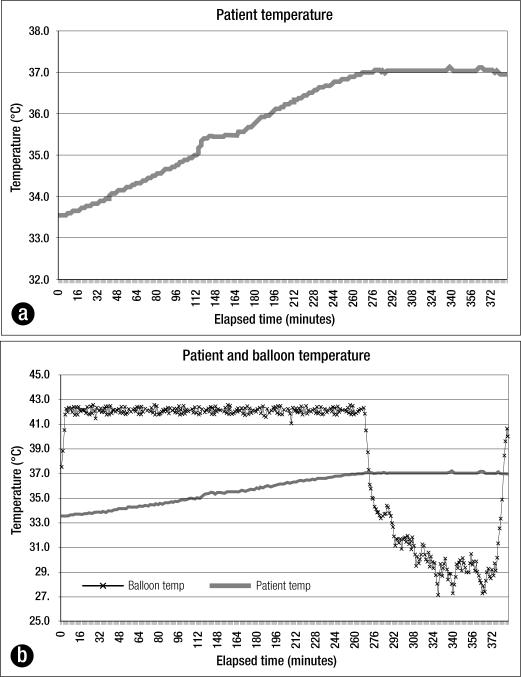
Sample temperature data plots of one of the patients, a 57-year-old man with stab wounds to the neck and abdomen, are shown in Figures 3a and 3b. On arrival to the ED, he had a core temperature of 33.3°C, systolic blood pressure of 65 mm Hg, pH of 6.99, and base deficit of 14. The patient's temperature rose from 33.6°C to 37.0°C over 4.5 hours (0.76°C/h) while in the OR undergoing emergent trauma laparotomy and neck exploration for hemorrhage control, necessitating 20 units of blood product transfusion.
Figure 3.
Trauma patient #66. (a) His temperature rose from 33.6°C to 37.0°C over 4.5 hours. (b) When the patient reached the target temperature, the machine decreased the fluid temperature in the balloon.
As the machine was left to automatically maintain the set temperature, the thermostat sensed that this patient had achieved the 37.0°C setpoint and decreased the fluid temperature in the balloon to prevent overwarming. Ten minutes later, when the patient's core temperature increased to 37.1°C, the system further reduced the balloon temperature to begin cooling the patient. The target temperature was automatically maintained for the following 2 hours by balloon temperature adjustments (Figure 3b).
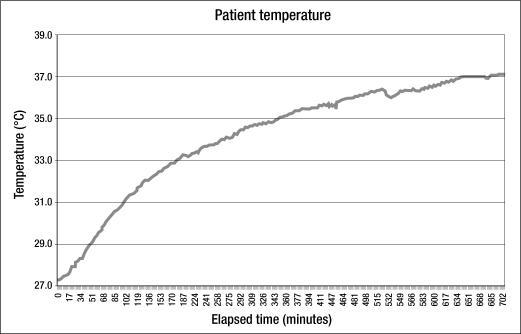
Figure 4 shows the rewarming curve of a 45-year-old patient treated for primary hypothermia. He was found down outdoors. On arrival to the ED, his core temperature was 25.2°C (77°F), and it rose to only 27.3°C over the next 2.5 hours (0.8°C/h) by use of a warm-air convection blanket and warm intravenous fluid administration. His core temperature reached 33.0°C after 3 hours of intravascular rewarming at an average warming rate of 1.9°C/h. The curve demonstrates how the warming rate slows as core temperature approaches 37°C, due to progressive heat distribution through peripheral tissues as vasoconstriction decreases.
Figure 4.
Rewarming in a patient with primary hypothermia.
Although none of the five survivors required treatment for bacteremia, two patients were treated successfully for ventila-tor-associated pneumonia. One of these also experienced a nonfatal pulmonary embolus, and subsequent diagnostic workup revealed a deep vein thrombosis at the femoral catheter site. This event occurred 1 week after the rewarming catheter was removed and replaced over a guidewire with a standard triple-lumen central venous catheter. The patient was treated with anticoagulation without additional complications.
DISCUSSION
The combination of hypothermia, coagulopathy, and acidosis in trauma patients is often referred to as the “triad of death.” The concept of “damage-control surgery” to reduce the operative time necessary to control bleeding and contamination was devised to minimize the development of the aforementioned lethal “triad” (20).
Hypothermia in critically injured patients may result from shock, environmental exposure, or both. Vasoconstriction due to surface exposure, volume loss, and catecholamine release reduces peripheral blood flow, which decreases oxygen consumption and radiant surface heat loss. The purpose of this process is to preserve central organ perfusion and heat and to centrally contain oxygen and energy sources for metabolism. When heat loss continues to the extent that core temperature falls, central organ metabolism and function decrease due to slowing of enzymatic reactions, reducing oxygen demand. As a result, cardiac output is diminished, reducing energy and metabolite circulation. Although reduction of metabolism slows the accumulation of oxygen debt, it will nonetheless continue to grow over time and “add insult to injury” in trauma patients.
In a retrospective analysis of matched groups of trauma patients, both injury severity by ISS and blood product transfusion volume were shown to correlate with degree of hypothermia. Severe hypothermia, in turn, independently increased mortality risk, as lower temperature nadir and longer duration of hypothermia were found to significantly increase risk of death (3, 4). The complex interactive effects of simultaneous injury, cold, and physiologic disturbance make it difficult to assign cause-and-effect relationships among them. However, if not corrected, each has a negative influence on survival. Furthermore, subsequent prospective, randomized investigations have demonstrated that rapid rewarming in this patient population does in fact reduce resuscitative volume requirements and perhaps mortality (7, 8).
Coagulopathy caused by clotting factor and platelet loss due to hemorrhage is potentially correctable with control of ongoing hemorrhage and replacement with blood product infusions. The dysfunction of platelets and the coagulation cascade in the hypothermic patient, however, will persist until normothermia is restored. Laboratory coagulation study samples are warmed to 37°C before testing; therefore, they reflect only the quantitative adequacy of the clotting factors present rather than the degree of qualitative coagulopathy due to reduced enzymatic activity at lower temperatures. Platelet counts likewise do not give any indication of the adequacy of platelet function. This further emphasizes the importance of rewarming in the acute phase of the treatment of trauma patients (9, 11).
Aggressive rewarming to achieve normothermia in severely injured patients is a critical component of improving coagulopathy and restoring normal physiologic function. Although it cannot take priority over surgical hemostasis and circulating volume restoration, these can and should all be addressed simultaneously.
Warming methods
Surface warming produces localized superficial vasodilation and redistribution of blood away from the central circulation, which results in accelerated core heat loss until surface temperature rises above core temperature. Adverse hemodynamic effects may also occur if the centralized circulating volume pool, generated by prolonged vasoconstriction, is lost too rapidly. Indeed, overaggressive superficial rewarming by warm water submersion can produce abrupt peripheral vasodilation with rapid circulating volume redistribution to peripheral tissues and cardiovascular collapse and is thus not recommended. Furthermore, a reperfusion syndrome may also develop after a prolonged period of relative peripheral tissue ischemia from recirculation of the products of hypoxic metabolism. External application of warm blankets, a warm environment (convective air blanket), and warm-air ventilation are important to reduce ongoing heat loss and should be a consistent practice. However, they are inefficient (<0.5°C/h) and inadequate to increase body temperature in the setting of decreased metabolic heat production, which is common in trauma patients and universal in shock. Warm-water circulating blankets may augment external rewarming efforts, but with only a negligible amount of additional heat transfer (6, 12).
Internal rewarming strategies are somewhat more effective. Resuscitation with warmed intravenous fluids and blood products, which are stored at 4°C, using an actively heating infusion system is essential to minimize iatrogenic, and thus preventable, decreases in core body temperature. At most the machines may warm fluids up to 40°C, which at resuscitation rates can add 1 to 1.5°C/h in patients who do not have ongoing heat loss. Pleural, pericardial, or peritoneal cavitary lavage can add 2 to 2.5°C/h but may be too invasive to justify their risk given the safer and more efficacious alternatives (6, 12).
Continuous veno-venous rewarming with a 40°C hemodiafiltration (CVVH) machine may produce core rewarming rates of up to 2°C/h in cases of primary (nontrauma) hypothermia but often requires a specially trained nurse or technician for monitoring (12–14). Continuous arteriovenous rewarming (CAVR) with a 40°C extracorporeal passive countercurrent warmer has been demonstrated specifically in critically injured patients to produce an average core rewarming rate of 2°C/h in the ICU setting. Gentilello and colleagues' prospective work on the subject has produced strong evidence that rapid extracorporeal rewarming reduces resuscitative volume requirements and perhaps mortality compared with conventional external rewarming measures. However, extracorporeal rewarming with the CAVR technique is still somewhat invasive, requiring both venous and arterial large-bore (8Fr) catheterization and sufficient systolic blood pressure (at least 80 mm Hg) to shunt blood through the warming apparatus (7, 8). Rewarming using a cardiac bypass pump has long been considered the “gold standard” technique; however, its invasiveness is similar to that of CAVR, it requires a trained nurse or technician to operate, and it requires heparin anticoagulation, which is not feasible in most trauma patients. Furthermore, all of the above forms of extracorporeal circulation carry the potential risk, albeit small, of catastrophic hemorrhage or air embolus if an opening in the circuit occurs. These three extracorporeal methods are the most rapid means of direct core rewarming described to date and are currently considered the standard techniques for appropriately selected and monitored patients, given the small amount of risk.
Intravascular rewarming
The intravascular location of the warming component has several potential advantages: it does not require arterial access or blood filters, does not depend on blood pressure as a driving force, and does not put the patient at risk of hypotensive events caused by extracorporeal circulation rate fluctuations or volume loss. There is also minimal risk of hemorrhage or air embolus since there is no direct contact with the intravascular circulation.
Since most severely injured patients undergo placement of central venous access catheters early in the course of their management, a rewarming balloon catheter may be alternatively placed in patients who are, or are likely to become, hypothermic. This may include severely injured patients who either arrive to the ED hypothermic, have lost enough blood to acutely require a large volume of resuscitation, or will require operative or angiographic intervention and will thus experience further heat loss from open body cavities and cold environments.
The intravascular system does not require a specially trained technician. The tubing circuit is easily and quickly assembled with minimal training, and once the target temperature is set, the system requires only minimal supervision. There are no filters to change or clog, and there is no temperature overshoot or fluctuation: once the target core temperature is reached, it is maintained within 0.2°C by heating or cooling as necessary. If, for example, intravenous fluids or blood products are administered, the computer will sense any decrease in core temperature and adjust to maintain the target temperature. The machine is small and lightweight and thus is easily moved with the patient from the ED to the angiography suite, OR, or ICU. Continuous rewarming therefore can occur early, when the patient is at greatest risk of both heat loss and the adverse consequences of coagulopathy. In contrast, in previous studies on the efficacy of extracorporeal rewarming, the technique was usually initiated later in the patients' course in the relative safety and warmth of the ICU.
The safety of any new device or technique in patient care must always be addressed. Insertion of central venous catheters carries several inherent risks, including placement errors, bleeding, infection, catheter-related venous thrombosis, pneumothorax, and injury to nearby structures. No definitive evidence has been presented to indicate any difference in complication rates between these balloon catheters and other central vein catheters. There is a unique need to investigate any potential thrombogenic effects of the warming balloons on the surface of the intravascular portion of the catheter. The incidence has been shown to not be different when the system is used for therapeutic cooling and subsequent rewarming of patients sustaining acute intracranial or cardiac events (15, 16). Therefore, it does not appear that the flow-impedance effect of the catheters' slightly larger cross-sectional area due to the balloons contributes to the local intravascular formation of clots. However, it remains to be clinically proven that warm (42°C) balloons do not increase the risk of surface clot formation.
According to the system's operation manual, any breaks in the closed tubing circuit are promptly sensed by the machine, flow is stopped, and alarms indicate the problem. If air enters the system it does not come into contact with the bloodstream and is removed continuously by the air trap. If a balloon breaks, a small amount of sterile normal saline may enter the patient's circulation before detection (10). Finally, as with any other invasive catheter, in order to reduce the risk of complications, the catheter should be removed promptly when no longer needed.
Once hemorrhage and contamination are controlled and the patient is rewarmed and resuscitated with correction of coagulopathy and acidosis, consideration can be given to instituting therapeutic cooling, if indicated, or fever control to minimize ongoing damage associated with central nervous system or myocardial insults using the same system already in place (17, 18).
The incidence of hypothermia (temperature ≤35°C) on arrival to the ED among all critically injured patients (ISS ≥ 25) admitted to our institution in Dallas, Texas, a relatively warm climate, over the past 8 years was 6.6%. Reports from other trauma centers indicate that, depending on climate, up to 40% of critically injured trauma patients may arrive at the hospital hypothermic, and the degree of hypothermia correlates well with the magnitude of injury (3–5). A large percentage of severely injured patients acutely undergo some type of invasive procedure, putting them at risk for additional heat loss due to environmental exposure. Specifically, 50% of patients leave the OR hypothermic (5). In our study group, the average duration of this exposure risk was 4.5 hours. Our initial experience using intravascular rewarming in critically injured patients has shown that even under conditions of significant ongoing heat loss, warming of up to 2°C/h may be achieved. In more controlled environments, such as the ICU, warming rates of 4°C/h or more may be possible. This technique may also be applicable to other situations such as primary hypothermia due to exposure or intraoperative warming of nontrauma patients with significant blood loss, transfusion requirements, and coexistent hypothermia (21). Another potential application exists with brain-dead organ donors who may lose thermostatic regulatory control due to hypothalamic failure (19).
CONCLUSIONS
Intravascular heat exchange with a balloon catheter provides rapid active core rewarming, even in patients with unavoidable ongoing heat loss. It is easily set up and initiated and carries no apparent additional risk over that of other central venous catheters. Once the desired core body temperature is set, the system continuously and automatically works to achieve and maintain it. Rewarming can be initiated in the ED and continued in the OR, interventional radiology suite, and ICU with minimal effort. Therefore, rewarming can occur simultaneously with resuscitation and hemorrhage control efforts.
Our experience with intravascular core rewarming and temperature maintenance in critically injured patients has been encouraging and warrants further investigation in a prospective fashion to study its effects on survival, coagulopathy, acidosis, and transfusion requirements. Its safety profile, with special attention to local catheter-related deep vein thrombosis, and its cost-effectiveness must also be evaluated.
References
- 1.American College of Surgeons . Advanced Trauma Life Support. 7th ed. Chicago: American College of Surgeons; 2004. pp. 240–241. [Google Scholar]
- 2.Danzl DF, Pozos RS, Auerbach PS, Glazer S, Goetz W, Johnson E, Jui J, Lilja P, Marx JA, Miller J. Multicenter hypothermia survey. Ann Emerg Med. 1987;16(9):1042–1055. doi: 10.1016/s0196-0644(87)80757-6. [DOI] [PubMed] [Google Scholar]
- 3.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27(9):1419–1424. [PubMed] [Google Scholar]
- 4.Luna GK, Maier RV, Pavlin EG, Anardi D, Copass MK, Oreskovich MR. Incidence and effect of hypothermia in seriously injured patients. J Trauma. 1987;27(9):1014–1018. doi: 10.1097/00005373-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Gregory JS, Flancbaum L, Townsend MC, Cloutier CT, Jonasson O. Incidence and timing of hypothermia in trauma patients undergoing operations. J Trauma. 1991;31(6):795–800. doi: 10.1097/00005373-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Gentilello LM, Pierson DJ. Update in nonpulmonary critical care: trauma critical care. Am J Respir Crit Care Med. 2001;163(3 Pt 1):604–607. doi: 10.1164/ajrccm.163.3.2004106. [DOI] [PubMed] [Google Scholar]
- 7.Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O'Keefe GE. Is hypothermia in the victim of major trauma protective or harmful? A randomized, prospective study. Ann Surg. 1997;226(4):439–449. doi: 10.1097/00000658-199710000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentilello LM, Cobean RA, Offner PJ, Soderberg RW, Jurkovich GJ. Continuous arteriovenous rewarming: rapid reversal of hypothermia in critically ill patients. J Trauma. 1992;32(3):316–327. doi: 10.1097/00005373-199203000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gubler KD, Gentilello LM, Hassantash SA, Maier RV. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994;36(6):847–851. doi: 10.1097/00005373-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 10.CoolGard 3000 Operation Manual Irvine, CA: Alsius Corporation, 2007.
- 11.Ferrara A, MacArthur JD, Wright HK, Modlin IM, McMillen MA. Hypothermia and acidosis worsen coagulopathy in the patient requiring massive transfusion. Am J Surg. 1990;160(5):515–518. doi: 10.1016/s0002-9610(05)81018-9. [DOI] [PubMed] [Google Scholar]
- 12.Vassal T, Benoit-Gonin B, Carrat F, Guidet B, Maury E, Offenstadt G. Severe accidental hypothermia treated in an ICU. Chest. 2001;126(6):1998–2003. doi: 10.1378/chest.120.6.1998. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu S, Shimomatsuya T, Kobuchi T, Nakajima M, Amaya H, Konishi S, Shiraishi S, Ono S, Maruhashi K. Severe accidental hypothermia successfully treated by rewarming strategy using continuous venovenous hemodiafiltration system. J Trauma. 2007;62(3):775–776. doi: 10.1097/01.ta.0000195446.00160.6c. [DOI] [PubMed] [Google Scholar]
- 14.Brauer A, Wrigge H, Kersten J, Rathgeber J, Weyland W, Burchardi H. Severe accidental hypothermia: rewarming strategy using a venovenous bypass system and a convective air warmer. Intensive Care Med. 1999;25(5):520–523. doi: 10.1007/s001340050891. [DOI] [PubMed] [Google Scholar]
- 15.Schmutzhard E, Engelhardt K, Beer R, Brossner G, Pfausler B, Spiss H, Unterberger I, Kampfl A. Safety and efficacy of a novel intravascular cooling device to control body temperature in neurologic intensive care patients: a prospective pilot study. Crit Care Med. 2002;30(11):2481–2488. doi: 10.1097/00003246-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32(2):559–564. doi: 10.1097/01.CCM.0000108868.97433.3F. [DOI] [PubMed] [Google Scholar]
- 17.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part I: indications and evidence. Intensive Care Med. 2004;30(4):556–575. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 18.Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11(4):R91. doi: 10.1186/cc6104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassin SL, Bleck TP, Nathan BR. Intravascular temperature control system to maintain normothermia in organ donors. Neurocrit Care. 2007 doi: 10.1007/s12028-007-9008-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Feliciano DV, Moore EE, Mattox KL. Trauma damage control. In: Moore EE, Feliciano DV, Mattox KL, editors. Trauma. 5th ed. New York: McGraw-Hill; 2004. pp. 877–900. [Google Scholar]
- 21.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71–77. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]