Fig. 1.
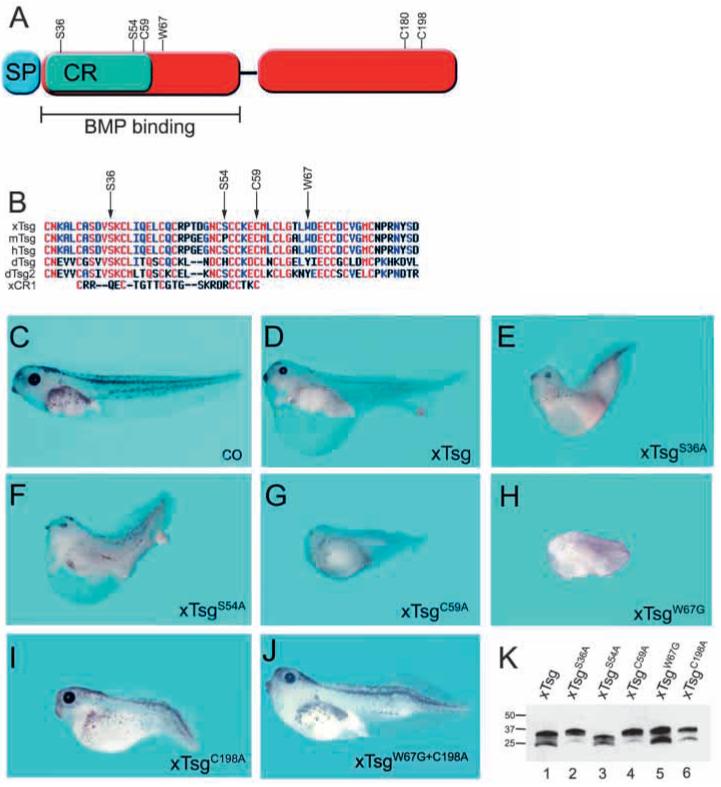
The ventralizing activity of Tsg resides in its C-terminal cysteine-rich domain. (A) The Tsg mutations presented here. (B) Alignment of the N-terminal cysteine rich domain of Tsg proteins from Xenopus (xTsg), mouse (mTsg), human (hTsg), Drosophila (dTsg, dTsg2) and Chordin CR1. (C-J) Stage 42 wild-type control embryo (C) and embryos radially microinjected at the four-cell stage with a total of 2 ng xTsg mRNA (D), xTsgS36A (E), xTsgS54A (F), xTsgC59A (G), xTsgW67G (H), xTsgC198A (I), xTsgW67G+C198A (J). Each construct was analyzed in at least three independent experiments with n>30. (K) xTsg and mutant xTsg proteins secreted by dissociated animal cap cells after microinjection of 2 ng of the indicated mRNAs.
