Fig. 2.
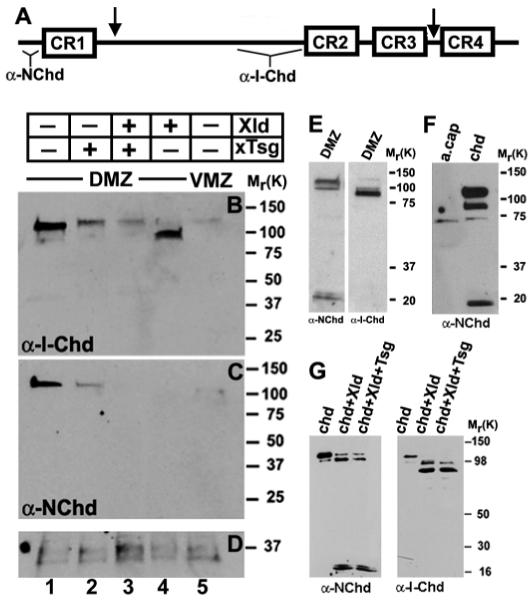
Tsg promotes the degradation of endogenous Chordin fragments. (A) Schematic representation of the cleavage sites of Xolloid in Chordin (arrows). The regions of Chordin protein used to generate the anti-N-Chd and anti-I-Chd antibodies are indicated. (B-D) Embryos were injected marginally into each blastomere at the four-cell stage with Xenopus Tsg (2 ng total, lane 2), Xolloid (0.8 ng total, lane 4) or both mRNAs (lane 3). Dorsal marginal zones (DMZ, lanes 1-4) or ventral marginal zones (VMZ, lane 5) were explanted at early gastrula (stage 10), cells were dissociated and the Chordin protein secreted during 3 hours analyzed in western blots using anti-I-Chd (B) or anti-N-Chd (C) antibodies. (D) Loading control showing a protein that crossreacts with the secondary antibody. (E) DMZs isolated at stage 11 and incubated for 12 hours at room temperature show the canonical Chordin degradation fragments but no additional products. (F) Proteins secreted by animal caps from uninjected (lane 1) and chordin-injected embryos (lane 2) were detected by anti-N-Chd immunoblot. Xenopus embryos were injected into the animal pole with 200 pg of chordin mRNA, ectodermal explants isolated at blastula stage, and dissociated cells incubated for 12 hours at room temperature. (G) Western blot analysis of Xenopus Chordin protein probed with anti-N-Chd or anti-I-Chd after digestion for 10 hours at room temperature with control medium (lane 1), Xolloid (lane 2), and Xolloid and Xenopus Tsg (lane 3). Note that the pattern obtained for Chordin digestion in vivo (E,F) is the same one as obtained after in vitro digestion of Chordin (G), and that additional proteolytic fragments were not observed in the embryo.
