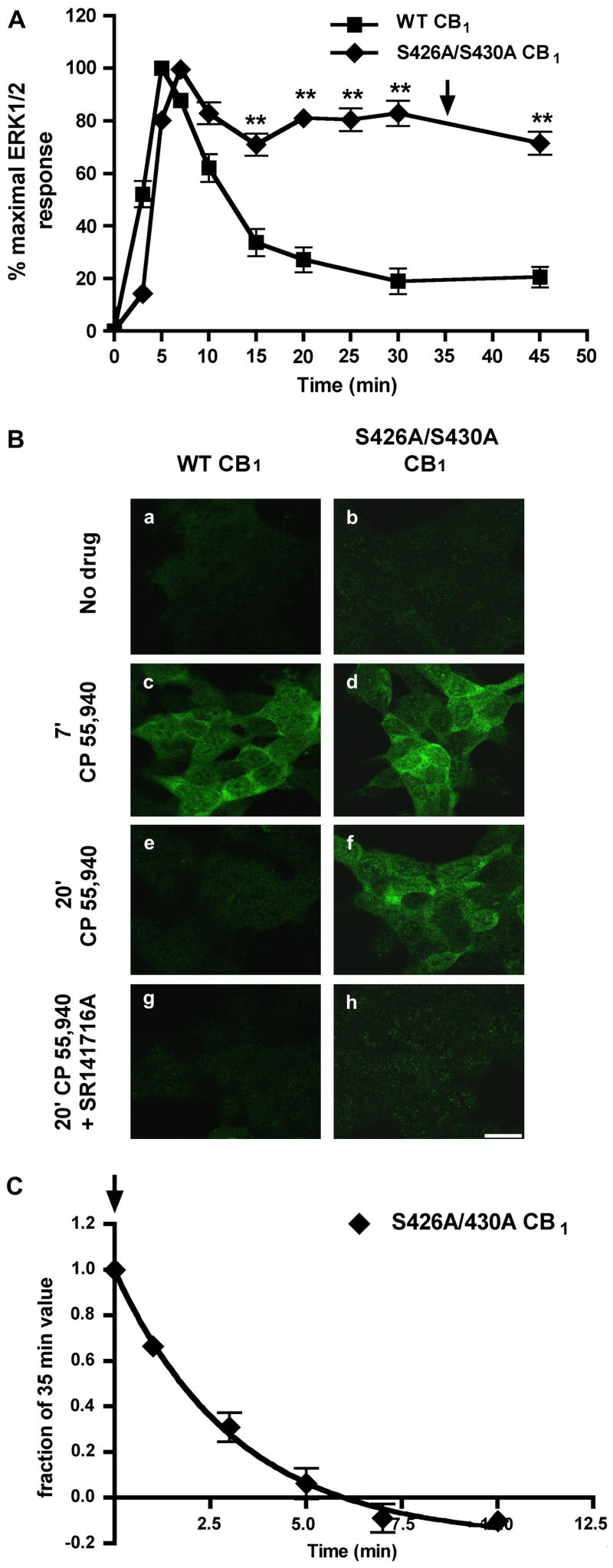
Fig. 1. The sustained ERK1/2 phosphorylation seen with S426A/S430A CB1 is mediated by continued agonist-induced activation.
(A) Quantitative detection of phospho-ERK1/2. HEK293 cells stably expressing either wild-type or S426A/S430A CB1 receptors were treated with 100 nM CP 55,940 for the indicated times and the time course of ERK1/2 activation (measured by ERK1/2 phosphorylation) was determined (see Methods). The S426A/S430A CB1 receptor-mediated activation of ERK1/2 is significantly prolonged. Data are mean ± SEM and were collected from 5 or more experiments performed in duplicate. **p<0.01 compared with individual wild-type time points by unpaired t-test. Arrow indicates where SR1 was added in (C). (B) Immunocytochemical detection of phospho-ERK1/2 in HEK293 cells stably expressing either wild-type or S426A/S430A CB1. Cells were treated with either vehicle (a–b), 100 nM CP 55,940 alone (c–f), or pretreated with 1 μM SR1 followed by application of 100 nM CP 55,940 in the presence of 1μM SR1 (g–h). Phospho-ERK1/2 was detected immunoctyochemically (see Methods). Both wild type and S426A/S430A CB1 increased phospho-ERK1/2 immunoreactivity at 7 min. However, with S426A/S430A CB1 substantial phospho-ERK1/2 immunoreactivity was still evident at 20 min. In contrast, with wild type CB1 phospho-ERK1/2 immunoreactivity was transient, returning to control levels after 20 min. Pre-treatment with SR1 blocked ERK1/2 activation by both receptors. Scale bar, 20 μm. (C) Prolonged activation of ERK1/2 by the S426A/S430A CB1 receptor was rapidly (t1/2= 2.97 min) reversed by addition of 1μM SR1 after 35 min of 10 nM CP 55,940 stimulation (time of SR1 addition indicated by arrow in A and C). Data are mean ± SEM with n = 6 from three experiments performed in duplicate.