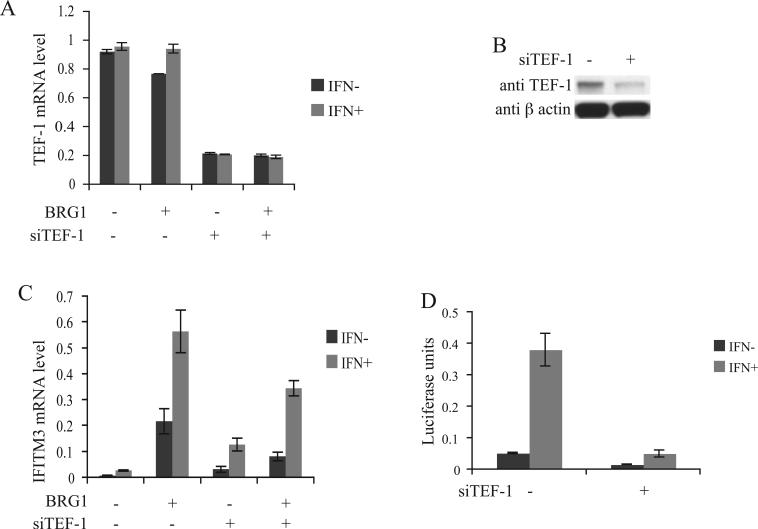
Fig.4.
TEF-1 knock-down results in the reduction of IFITM3 promoter activity and expression levels. (A) SW-13 cells were transfected with siTEF-1 RNA oligonucleotides or the control RNA oligonucleotides and co-transfected with either pREP7-BRG1-Hygromycin or pREP7-Hygromycin control vector. Total RNAs were isolated from the cells selected with hygromycin for 48 h and TEF-1 mRNA levels were determined using quantitative real-time PCR. The cells were induced with 1000 u/ml α-interferon (IFN-α) for 12 h. (B) Western blot showing the TEF-1 levels in SW-13 cells transfected with siTEF-1 RNA oligonucleotides or the control RNA oligonucleotides and co-transfected with pREP7-Hygromycin. Protein was isolated from the cells selected with hygromycin for 48 h. β actin was used as control (C) IFITM3 mRNA levels in SW-13 cells. Conditions same as in figure 4A. (D) IFITM3 promoter activity in the wild-type and TEF-1 knock-down HeLa cells. pREP4-TM3-Luc was co-transfected into HeLa cells with siTEF-1 RNA oligonucleotides or the control RNA oligonucleotides. The cells were induced with 1000 u/ml IFN-α for 12 h. Luciferase activity was measured 48 h after transfection.