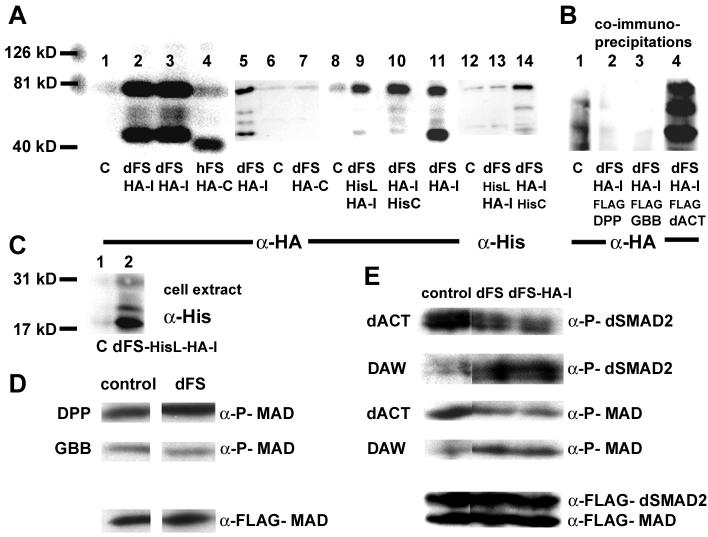
Fig. 2. Functional analysis of dFS in S2 cells.
The molecular markers (126, 81, and 40 kD) are used to determine the size of proteins in (A) and (B). (A) Lanes 1-4, 5-7, 8-11, and 12-14 are independent experiments. (A, lanes 1, 6, 8, and 12) Supernatant from mock-transfected S2 cells. Unlike hFS (A, lane 4), dFS is processed (lanes 2, 3, 5, 11). On western blots, internally tagged dFS-HA-I is detected in the supernatant as two major bands of approximately 75 kD and 45 kD. The upper band is larger than 62 kD, the expected size of the processed form without the signal peptide. Differences in size are likely due to glycosylation. Two minor additional processed forms migrate at about 55 kD. (Lane 4) The C-terminally tagged human FS migrates at approximately 40 kD, about 10 kD higher than expected. (Lane 7) In contrast to hFS-HA-C, a C-terminally tagged dFS cannot be detected on western blots. (Lane 14) Similarly, a C-terminally His/internally HA-double-tagged dFS shows a robust signal with anti-HA but only a weak signal with anti-His antibodies. These results indicate that the C-terminus is removed. (B) In co-immunoprecipitation experiments with FLAG tagged DPP, GBB, and dACT, we can detect all processed forms of dFS-HA-I in the presence of dACT. However, none of the forms co-immunoprecipitates in the presence of DPP or GBB. (C) To study N-terminal processing of dFS, amino acids 132-141 (70-78 in our shorter construct) in dFS-HA-I were replaced by a His-epitope. The His-tag is recognized in cell extracts as an 18 kD protein (lane 2). In the supernatant, this protein is detected by anti-HA but not anti-His antibodies (A, lanes 9 and 13). (D and E) Ligand signaling and phosphorylation of SMADs: Supernatants of dFS or internally tagged dFS-HA-I expressing cells were pooled, divided, and mixed with supernatant of mock or ligand transfected cells and added to cells that over-express FLAG-MAD and dSMAD2. (D) dFS levels that can inhibit dACT do not inhibit DPP and GBB signaling. (E, row 1 and 3) dFS and dFS-HA-I inhibits dACT-mediated activation of dSMAD2 and MAD. (Row 2 and 4) dFS increases rather than decreases phosphorylation mediated by DAW.