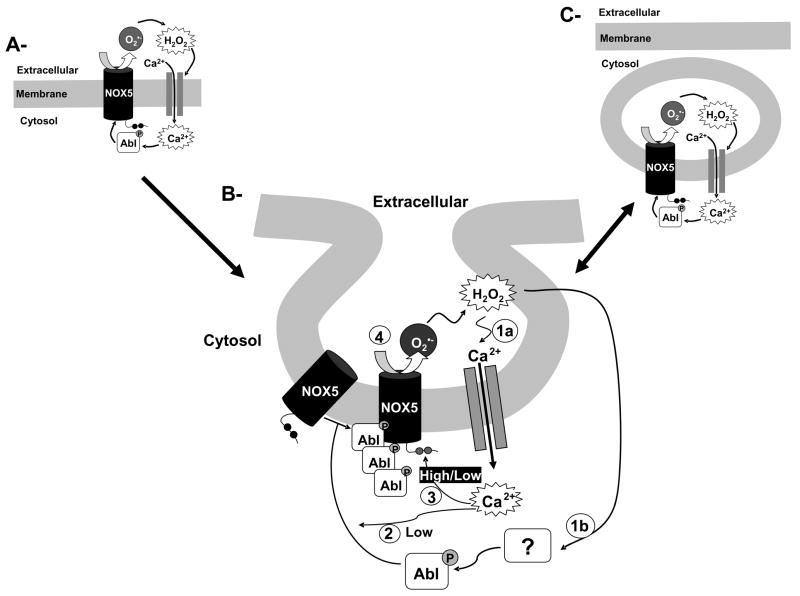
Fig. 9. Proposed model for H2O2-NOX5 regulation: the amplification loop.
H2O2 induces simultaneously a low calcium influx (panel B, step 1a) and the activation of cAbl through tyrosine phosphorylation (panel B, step 1b). The low calcium influx and the activation of c-Abl are required for the translocation of c-Abl to the membrane (panel B, step 2). These events are accompanied by c-Abl oligomerization. The c-Abl oligomers through either a direct or indirect interaction with NOX5 stimulate its activity, possibly by enhancing its sensitivity to a low concentration of calcium (panel B step 3). Once NOX5 is activated, the generation of superoxide anion through its dismutation to hydrogen peroxide and/or through the formation of more highly reactive oxygen species then amplifies the primary signal (panel B, step 4). When a stimulus generates a high cytosolic calcium concentration (e.g. ionophore exposure), the stimulation of NOX5 is independent of c-Abl, and Ca2+ then directly activates NOX5. Along with this amplification loop there is evidence suggesting the compartmentalization of superoxide production, which occurs only at the plasma membrane and in discrete intracellular vesicular compartments networking with the plasma membrane (panel A, B and C and Fig. 5A). However, our results suggest that the initial event in this pathway is the production of superoxide at the plasma membrane.