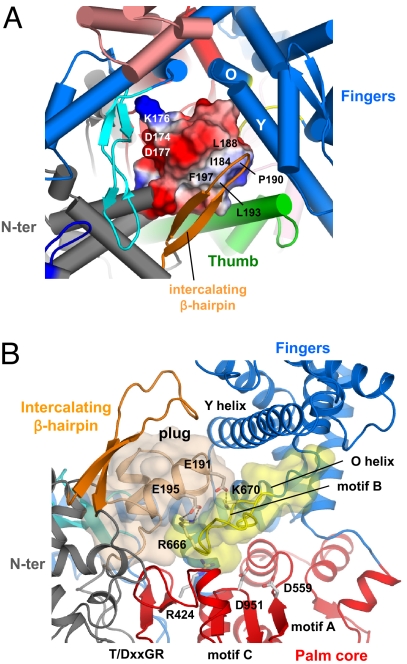
Fig. 3.
The Plug module blocks the DNA-binding channel. (A) Close-up view of the DNA-binding channel of N4 mini-vRNAP shown in the same orientation as Fig. 1A Right. The plug module, which has an amphipathic surface, is depicted as its molecular surface with electrostatic distribution (positive electrostatic potential is blue, negative potential is red, and neutral is white). The hydrophobic surface (including residues I184, L188, P190, L193, and F197) interacts with the intercalating β-hairpin and Thumb, whereas the hydrophilic surface (including residues D174, K176, and D177) faces the Palm core. (B) Close-up view showing that the plug and the Palm core sandwich the motif B loop, burying almost all amino acid residues essential for the catalytic activity of RNAP; R424 (T/DxxGR motif) for substrate binding, D559 (motif A), and D951 (motif C) for chelating the catalytically essential Mg2+ ions, and R666 and K670 (motif B) for substrate binding. E191 and E195 in the plug form salt bridges, indicated as dotted lines, with R666 and K679, respectively. The Thumb and Palm insertions have been removed from this view for clarity.