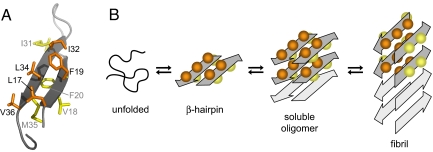
Fig. 4.
Hypothetical aggregation mechanism involving the Aβ β-hairpin. (A) β-hairpin with the interior hydrophobic core side chains (orange) and the exterior side chains (yellow). (B) Schematic of a hypothetical aggregation mechanism that involves the β-hairpin as a transiently populated conformation sampled by the disordered monomer and/or as a constituent of oligomeric Aβ. Soluble oligomers form by hydrophobic stacking of β-hairpins. A concerted conformational transition establishes a fibril seed with in-register, parallel β-sheets. The side chains incorporated in the hydrophobic core within one molecular layer of Aβ(1–40) fibrils correspond to the interior side chains in the ZAβ3:Aβ(1–40) complex. The mechanism is suggested based on the accessibility of the Aβ β-hairpin reported here and ATR-FTIR data reported in reference 35. However, it remains to be proven experimentally.