Abstract
Peritoneal cavity (PerC) B-1 cells have long been known to express CD11b, which is coexpressed with CD18 to form the Mac-1/CR3 complement receptor and adhesion molecule. However, although all PerC B-1 cells are commonly believed to express CD11b, we show here that nearly half of the cells in each of the PerC B-1 subsets (B-1a and B-1b) do not express this surface receptor. The CD11b+ cells in each B-1 subset are larger and more granular and express higher levels of surface IgM than the CD11b− B-1 cells. In addition, the CD11b+ B-1 cells initiate the formation of tightly associated doublets that are present at high frequency in adult PerC. Finally, and most importantly from a developmental standpoint, the CD11b+ B-1 cells have a limited reconstitution capability: when sorted and transferred into congenic recipients, they reconstitute their own (CD11b+) B-1 subset but do not reconstitute the CD11b− B-1 subset. In contrast, CD11b− B-1 cells transferred under the same conditions efficiently replenish all components of the PerC B-1 population in appropriate proportions. During ontogeny, CD11b− B-1 cells appear before CD11b+ B-1 cells. However, the clear phenotypic differences between the neonatal and adult CD11b B-1 subsets argue that although CD11b− B-1 give rise to CD11b+ B-1 in both cases different forces may regulate this transition.
Keywords: CD5, Mac-1, B cell development, neonatal, IgM
In early studies, we identified a functionally distinct, self-replenishing murine B cell subset (B-1) that includes only a small proportion of the B cells in spleen but constitutes the ≈80% of the B cells in the peritoneal cavity (PerC). Shortly thereafter, we divided the B-1 subset into a majority population (B-1a) that expresses low, but clearly detectable, levels of cell surface CD5 and a minority “sister” population (B-1b) that has the same surface phenotype as B-1a except that it does not express CD5 (1–4). Cell transfer studies then showed that these populations, also referred to as subsets, are developmentally distinct, in that, each is capable of fully replenishing its niche when sorted and transferred to adoptive recipients and neither has the capability to more than minimally replenish the other (2, 5, 6). Consistent with this developmental distinction, and with differences noted between the B-1a and B-1b repertoire, recent studies have shown that B-1a and B-1b have distinct immune functions and respond differently to antigenic stimulation (1, 7–12).
PerC B-1a and B-1b have long been known to express low levels of CD11b (13, 14), a cell surface marker expressed at high levels on macrophages and monocytes. However, early attempts to determine whether all, or only the majority, of PerC B-1 cells express CD11b were confounded by difficulties in detecting the low CD11b expression levels on the B-1 cells. In essence, although some B-1 cells always fell below the threshold for detectable CD11b expression, the frequency of these putatively negative cells varied with the brightness of the fluorochrome-coupled reagent used to detect CD11b. Furthermore, regardless of the reagent used, there was no clear boundary separating the negative from the positive cells detectable with the technology available at the time.
Eventually, the lack of a clear distinction between CD11b+ and CD11b− cells, coupled with a tendency to simplify phenotypes in publications, led to the generally accepted idea that all B-1 cells express CD11b. Thus, not surprisingly, when Rothstein and colleagues (15) recently “recognized” that CD11b− B-1 cells are routinely present in the PerC, they classified these cells as a novel B-1 subset (B-1c). However, the independently initiated studies presented here demonstrate a clear developmental relationship between the CD11b− and CD11b+ B-1 subsets in adult PerC in that the CD11b− B-1 subset is both capable of reconstituting itself in adoptive recipients and differentiating to reconstitute the CD11b+ subset.
The reasons for the initial confusion vis-à-vis CD11b expression trace to limitations in the FACS technology available when CD11b (also colloquially referred to as Mac-1) was first detected on B-1 cells. The available FACS methods that were in use (and are still in use in many laboratories) introduce distortions that prevent the resolution of cells that are dully stained from those that are autofluorescent (see review in ref. 16). In addition, limitations in the number of available fluorescence “colors” in the early days restricted the ability to examine the expression of CD11b in combination with the markers necessary to fully define the B-1 subsets. Given these constraints, the best that could be said at the time was that the majority of cells in both the B-1a and B-1b subsets express CD11b. Nevertheless, although we were aware of the CD11b detection problem, simplification took over and we and others adopted the habit of calling all PerC B-1a and B-1b as CD11b+.
Studies presented here correct this error. By applying modern high-dimensional (Hi-D) FACS data collection and analysis methods (16, 17), we clearly resolve a CD11b+ PerC B-1 subset from a CD11b− PerC B-1 subset. By and large, the surface marker expression profiles of these two subsets, and their relative frequencies among B-1a and B-1b cells, are equivalent. Nevertheless, there are key differences between the CD11b− and CD11b+ subsets.
Most importantly, as we show here, CD11b− and CD11b+ B-1 cells differ sharply in their reconstitution capabilities. When sorted and transferred into intact allotype congenic hosts, CD11b+ cells reconstitute only their own (CD11b+) subset. However, CD11b− cells reconstitute both the CD11b− and the CD11b+ subsets in appropriate proportions, indicating a striking directionality in the reconstitution potential of the two subsets.
We also demonstrate the sequential appearance of CD11b− and CD11b+ cells during ontogeny, but identify phenotypic differences that distinguish these neonatal B-1 subsets from their apparent adult counterparts. Thus, we conclude that the CD11b− B-1 subset in adults does not simply contain persistent neonatal B-1 cells.
Finally, we demonstrate that CD11b+ B-1 cells have the curious ability to initiate the formation of tightly associated doublets that appear during ontogeny when CD11b+ B-1 cells develop and are present at high frequencies in adult PerC.
Results
CD11b Expression Subdivides Peritoneal B-1 Cells.
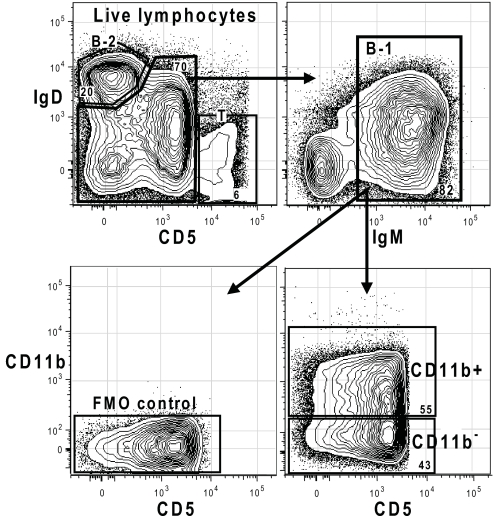
A little more than half of the B-1 cells in the PerC of adult BALB/c mice express surface CD11b. B-1 cells are identified by sequential gating of live IgMhighIgDlow lymphocytes (Fig. 1). The highest level of nonspecific staining and autofluorescence that can be expected on the gated B-1 cells is defined by the upper bound of the fluorescence-minus-one (FMO) control (Fig. 1 Lower Left), which shows the B-1 fluorescence in the CD11b channel for cells stained with all reagents but CD11b. CD11b+ cells within the gated B-1 population are identified as those cells whose CD11b levels are above the FMO threshold (Fig. 1).
Fig. 1.
Successive gating scheme for identifying the CD11b+ and the CD11b− subset on mouse B-1 cells. Total BALB/c adult PerC cells were stained according to the 11-color stain combinations described in Materials and Methods. Live cells were gated to include only lymphocytes (FSclow, SSclow), for which data are shown. The full gating sequence is included for on-line viewing.
The expression of CD11b on B-1 cells is ≈40-fold lower than on macrophages (data not shown) and is similar on those B-1a (CD5+) and B-1b (CD5−) cells on which it is expressed (Fig. 1). Thus, CD11b expression levels distinguish macrophages from CD11b-expressing B-1 cells in PerC and distinguish these B-1 cells from the CD11b− B-1 (B-1a and B-1b) subset, which fall into in a heterogeneous overall CD11b− PerC population that also contains B-2 and T cells.
CD11b+ and CD11b− B-1 Cells Are Phenotypically Distinct.
A series of phenotypic differences distinguish the CD11b+ B-1 subset from the CD11b− B-1 subset (Table 1). Notably, CD11b+ B-1 cells express more surface IgM and less surface IgD than CD11b− B-1 cells. In addition, as indicated by forward- and side-scatter measurements, CD11b+ B-1 cells are larger and more granular than CD11b− B-1 cells. These findings, coupled with the additional phenotypic differences shown in Table 1, reveal significant differences in gene expression between the CD11b− and the CD11b+ B-1 subsets in PerC. However, at present, the CD11b marker still remains the most efficient way of tracking the two B-1 subsets.
Table 1.
CD11b+ and CD11b− B-1 subsets have different surface phenotypes
| Surface marker | MFI |
|
|---|---|---|
| CD11b− B-1 cells | CD11b+ B-1 cells | |
| CD11b | = FMO | 3,080 |
| IgM | 7,100 | 13,600 |
| IgD | 1,300 | 890 |
| CD19 | 5,790 | 7,040 |
| B220 | 1,390 | 1,530 |
| CD5 | 2,170 | 2,020 |
| CD21 | 1,020 | 790 |
| CD23 | 500 | 300 |
| Gr-1 | = FMO | = FMO |
| Forward scatter | 830 | 1,000 |
| Side scatter | 2,260 | 2,760 |
Values represent the median of fluorescence intensity (MFI) found for each surface marker for B-1 cells isolated from adult BALB/c PerC. = FMO indicates that staining was coincident with staining obtained for the FMO control in which all reagents except the indicated reagent were present during the staining procedure. To identify the B-1 subsets, total PerC cells were analyzed according to the sequential gating scheme shown in Fig. 1. Data shown are representative of MFI acquired in >10 experiments. CD23 MFI shown for CD11b+ B-1 (300) is just above FMO.
CD11b− B-1 Cells Are Progenitors of CD11b+ B-1 Cells.
B-1 cells develop quite differently from B-2 cells (2, 14). Most, if not all, B-1 progenitors arise during fetal and neonatal development via pathways that are distinct from the bone marrow developmental pathway responsible for the development of most, if not all, B-2 cells (18–20). In adults, B-1 cells maintain their frequency and repertoire mainly or perhaps exclusively via self-replenishment (21). Thus, many studies have shown that the PerC B-1 cells of the adult mice contains stable, self-replenishing populations of B-1 cells that persist as such at least until the mice reach 6–9 months of age.
The biological mechanisms underlying this self-replenishment capability, and consequently the developmental processes that B-1 cells undergo to keep their numbers and repertoire in the PerC, is still unclear. Our finding that roughly half of B-1 cells in the PerC do not express CD11b, which is both a complement receptor and an important adhesion molecule, raised the question of whether CD11b expression could reflect differences in the self-replenishment capacity of B-1 cells in the PerC.
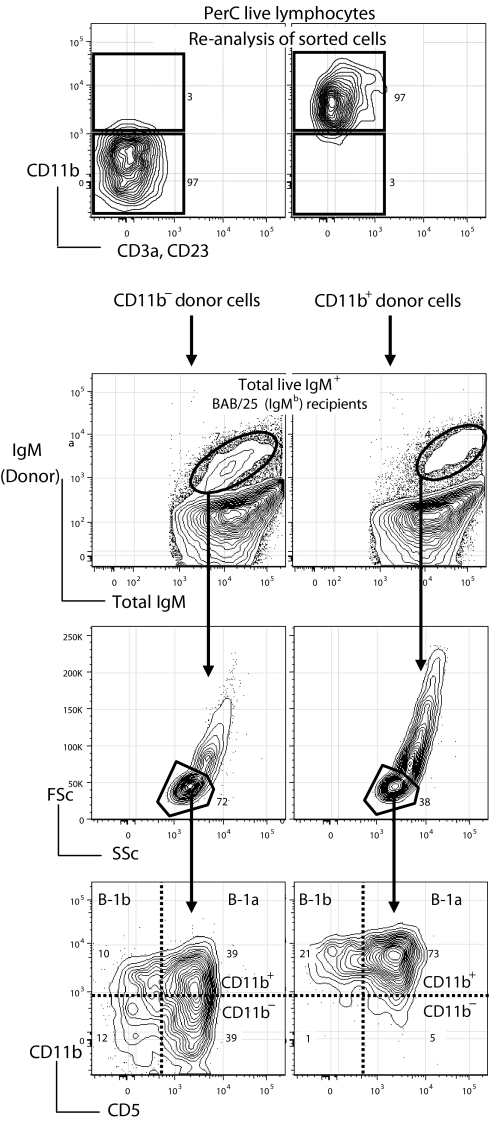
To evaluate this hypothesis, we sorted PerC CD11b− and CD11b+ B-1 subsets and transferred each into nonirradiated allotype congenic mice. Surprisingly, we found that CD11b− B-1 reconstitute all (known) peritoneal B-1 cells subsets at their typical frequencies in PerC, whereas CD11b+ B-1 only reconstitute their own CD11b+ subset (Fig. 2). Consistent with the differentiation of CD11b− B-1 to CD11b+ B-1 in transfer recipients, B-1 cells expressing CD11b are not detectable in the recipients until 2–3 days after transfer, when cells with low levels of CD11b expression appear (data not shown). Levels of CD11b then continue to increase, as does the number of CD11b+ B-1, until stable adult levels are reached by 10–12 days after transfer (Fig. 2).
Fig. 2.
CD11b− cells give rise to CD11b+ B-1 cells in adoptive recipients. CD11b− and CD11b+ PerC cells were sorted from BALB/c (IgMa allotype) as described in Materials and Methods. Each sorted population was transferred into the PerC cells of BAB/25 recipients (nonirradiated). At 10–12 days after transfer, total recipient PerC cells were harvested and stained. For analysis, live cells were initially gated to include only IgM+ cells and donor (IgMa) B cells were distinguished and gated by staining with a mAb that detects IgMa (IgH-6a), whereas recipient B were identified and gated with a mAb that detects IgMb (IgH-6b). Each B cell population was then further gated by size to include only cells within the lymphocytes scatter gates (FSclow/SSclow) and analyzed for the expression level of CD11b. Only B-1 cells were detected in the donor B cell population.
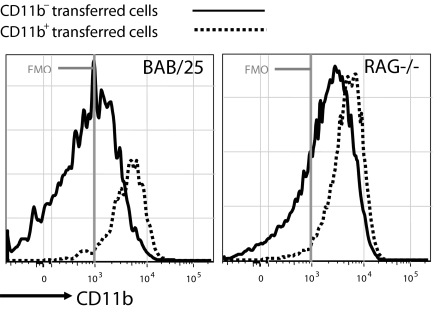
Transfers to RAG-deficient mice yield similar results, the only difference being that the CD11b is expressed at higher levels on the CD11b+ B-1 subset (Fig. 3). The reasons for this difference are unclear but may reflect conditioning of the environment by the previous presence of lymphoid cells in the intact host. Alternatively, it could reflect still further heterogeneity in the peritoneal B-1 population.
Fig. 3.
CD11b is expressed at higher levels on the CD11b+ B-1 cells than in RAG-deficient PerC recipients. CD11b− or CD11b+ donor cells were sorted and transferred into RAG−/− (nonirradiated) recipients. After 10–12 days, total recipient PerC cells were harvested, stained, and analyzed according to the procedures described for Fig. 2.
Collectively, results from these transfer studies establish that the CD11b− B-1 subset contains progenitors for the CD11b+ B-1 subset and show that the reverse is not true.
Doublet Formation by CD11b+ B-1 Subset in PerC.
CD11b+ B-1 has a curious tendency to form doublets and some larger aggregates in which the cells are firmly attached to each other. The light-scatter phenotype of the CD11b+ B-1 doublets (FSchigh and SSchigh) places them in the lower range of the “large cell” scatter gate, which also tends to include some monocytes and macrophages. B-1 cells found within this gate have usually been considered simply to be large, perhaps activated, B-1 cells. However, careful analysis shows that they are actually doublets containing two ordinary-size B-1 cells together that express roughly twice as much of the various surface markers found on individual B-1 cells.
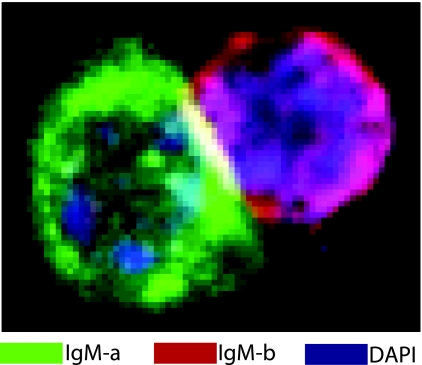
Studies with PerC from IgH allotype congenic, e.g., (BALB/cxCB.17)F1, show that roughly half of the B-1 cells in the large cell gate exist as doublets in which the two cells express different parental allotypes (one expressing IgMa and one expressing IgMb), whereas the remainder of the cells in the gate are doublets in which both cells express the same parental allotype (IgMa or IgMb). FACS sorting followed by confocal microscopy confirm that the large cells within this gate are doublets bound to each other sufficiently strong to resist shearing during sorting (Fig. 4) These doublets are detectable by microscopy even when directly isolated from the PerC and sorted, without staining or centrifugation, simply as cells that fall within the appropriate size gate (E.E.B.G., Leonard A. Herzenberg, and Takeshi Fukuhara, unpublished work).
Fig. 4.
CD11b+ B-1 cells form firmly attached doublets. Total PerC cells from adult (BALB/c × CB.17)F1 mice (IgMa x IgMb) were harvested and stained according to the procedures described in Materials and Methods. Because the IgM on the B-1 cells in F1 animals is encoded either by the parental IgMa chromosome or the parental IgMb chromosome, cells were stained with both anti-IgMa (FITC) and anti-IgMb (Texas red) reagents in addition to an anti-IgM reagent (used for sorting) that detects both IgMa and IgMb. For confocal microscopy, CD11b+ B-1 cells (CD19high, IgMhigh) that fell within the FSchigh gate were sorted onto coverslips in a 24-well plate. The sorted cells were prepared and analyzed by confocal microscopy as described in Materials and Methods.
The CD11b+ B-1 cells appear to be required to initiate or maintain doublet formation, because all doublets detected express CD11b. Furthermore, doublets are rare whenever the CD11b+ B-1 are rare, e.g., in CD11b− B-1 transfer recipients before the appearance of progeny CD11b+ B-1 (Fig. 2, light scatter plots), in spleen in unstimulated animals (data not shown), and, during early ontogeny before CD11b+ B-1 appear (see below). It is not clear whether both of the cells in a doublet have to express CD11b. However, at least one must do so.
The function of the doublets is also unclear at present. Nevertheless, their ubiquitous presence and consistent frequencies in various mouse strains at various ages strongly suggest that they are an important component of the immune system. This, in turn, suggests that CD11b expression on B-1 cells marks a functionally significant subset of these cells in the PerC.
In our studies here, and in most other modern studies, the B-1 doublets tend to be excluded by gating procedures instituted to exclude monocytes, macrophages, and nonspecifically aggregated cells. The impact of this gating procedure for functional studies is unclear. However, at a minimum, it interferes with the ability to estimate the total number of PerC B-1 cells, which is clearly larger than previously thought.
CD11b Expression Is Acquired Late in B-1 Ontogeny.
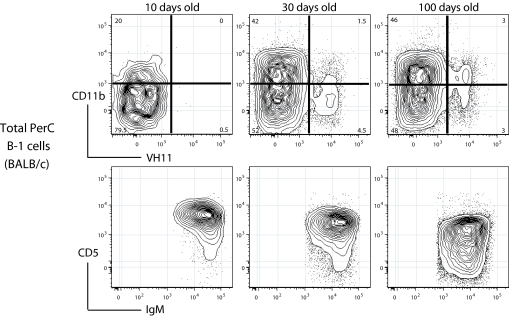
Ten days after birth (10d), BALB/c PerC contains ≈106 B-1 cells. This number increases gradually and reaches its stable adult level at 2–3 × 106 in 2–3 (100d) months later. The frequency of CD11b+ cells rises within this developing B-1 population: only ≈20% of the cells express CD11b at 10d, whereas CD11b+ B-1 cells comprise roughly half of the PerC B-1 population at 100d and maintain this frequency thereafter (Fig. 5 Upper). Thus, the earliest-appearing B-1 population is highly enriched for the CD11b− B-1 subset.
Fig. 5.
CD11b+ B-1 cells arise late in ontogeny but still express the typical neonatal surface phenotype, which only gradually shifts to the adult B-1 phenotype. Total PerC cells were harvested from BALB/c mice at different ages (neonates, 10 days, 30 days, and 100 days) and stained with an 11-color combination that simultaneously detects surface expression of CD11b, IgVH11, IgM, and CD5 (see Materials and Methods). B-1 cells were identified according to the sequential gating scheme shown in Fig. 1. (Lower) IgM and CD5 expression on the B-1 subset is shown. (Upper) CD11b and VH11 expression on the same cells is shown.
This CD11b expression pattern is reproduced during the development of B-1 cells that express IgH variable regions encoded by VH11, a VH gene uniquely expressed by B-1 cells and encoding antibodies that react with phosphatidylcholine. Previous studies have shown that B-1 cells expressing VH11 arise later during ontogeny than most other B-1 cells and only reach adult frequencies in mice >1 month of age (22–25). We confirm these findings here and show further that the earliest-appearing IgVH11-expressing B-1 cells are largely CD11b− (≈80%). Basically, we find that the development of these B-1 cells mirrors the overall development of B-1 cells but is offset by ≈20 days with respect to maturation to CD11b+ B-1 (Fig. 5 Upper).
The Predominant Phenotype of CD11b B-1 Subsets Shifts During Ontogeny.
IgM and CD5 expression levels are higher on the earliest-appearing B-1 cells than on B-1 cells in adults. This difference is visible in both the CD11b+ and the CD11b− subsets. In the overall B-1 subset, IgM is highest at 10 day, is intermediate at 30 days, and reaches the stable adult level by 100 days (Fig. 5 Lower). Importantly, in the VH11-expressing B-1 subset, this shift occurs ≈20 days later. Thus, the downward shift in IgM and CD5 expression, like the emergence of CD11b+ cells within the developing B-1 population, reflects ontological timing rather than responses to environmental changes.
Discussion
Studies here demonstrate clearly that B-1 cells in the mouse PerC are divided between two subsets, one of which expresses CD11b, whereas the other does not. In addition, we have shown that CD11b expression subdivides adult PerC B-1 cells into sequential developmental subsets: a CD11b− subset capable of reconstituting all peritoneal B-1 populations in their appropriate proportions, and its progeny CD11b+ subset, which only reconstitutes itself. Within the confines of the data, there is still room for CD11b+ B-1 cells to become or give rise to CD11b− B-1 in response to antigen or over a longer time than encompassed by our studies. In any event, the findings we have presented clearly indicate that the CD11b− cells have a substantially greater capability to differentiate to CD11b+ cells than vice versa.
The CD11b developmental subset distinctions cut across the earlier subdivision of B-1 cells into the B-1a and B-1b, now known to be functional subsets that respond to different antigenic stimuli. CD11b− and CD11b+ subsets are represented at equal frequencies in both the B-1a and B-1b subsets. Rothstein and colleagues (15) have suggested that CD11b− B-1 cells constitute a separate subset and have proposed naming this subset B-1c. However, the presence of CD11b− and CD11b+ cells within each of the existing B-1 subsets, and the developmental relationships between CD11b− and CD11b+ B-1 cells, argue strongly against defining the CD11b− cells as a separate (B-1c) subset on a par with B-1a and B-1b. Instead, we submit CD11b+ and CD11b− B-1 cells constitute developmental and functional subdivisions within the existing B-1a and B-1b subdivisions.
For the moment, we suggest referring to the CD11b subsets within the B-1a and B-1b subdivision simply as CD11b− and CD11b+, because CD11b expression is currently the most useful marker for identifying and sorting these cells. We have already identified additional surface markers that distinguish the CD11b subsets, which may well turn out to be distinguishable by still other phenotypic and functional differences that substantially surpass CD11b in terms of use as a defining marker. Thus, it seems prudent to wait before deciding on a definitive notation.
The functions that distinguish CD11b− B-1 from CD11b+ B-1 in adults have yet to be clearly understood. We have recently shown that LPS stimulates migration of PerC B-1 to the spleen and that all of the recent immigrants are CD11b+ (26). Because this migration occurs very rapidly, it is highly likely that the splenic immigrants originate from the CD11b+ PerC B-1 subset.
Once the immigrants enter the spleen, they gradually decrease CD11b expression and ultimately become CD11b− by our current definition. In some immigrants, this decrease in CD11b expression accompanies the division and differentiation of the immigrant cells to plasma cells (26). However, many of the immigrants simply divide and lose CD11b expression, and thus merge into the resident splenic B-1 population. It is possible that the immigrants later migrate back to the PerC, where they join the CD11b− subset and ultimately reinitiate CD11b expression to join the CD11b+ B-1 subset. Because the immigrant B-1 cells can divide in the spleen, this B-1 cycling from PerC to spleen back could be central to the continued self-replenishment of the B-1 population.
It is not surprising that CD11b is expressed on the B-1 cells that migrate from the PerC to the spleen. CD11b is on the α-chain integrin that associates with the β-chain CD18 to form the Mac-1/CR3 receptor. In addition to its role as a complement receptor, Mac-1 is an important adhesion molecule that binds preferentially to intercellular adhesion molecules and is known to participate in the cell migration. Thus, the differentiation of CD11b− to CD11b+ B-1 subset is likely to represent the developmental preparation of a population of cells that is ready to migrate to spleen and other sites of antibody production in response to appropriate stimuli. In any event, the demonstration that CD11b expression distinguishes functional and developmental subsets of PerC B-1, and their migratory brethren adds a new facet to the already complex mechanisms involved in enabling and implementing innate immunity.
Materials and Methods
Mice and Tissue Preparation.
BALB/c, BAB/25, and RAG1-deficient mice were bred and maintained at the Department of Comparative Medicine at Stanford University. PerC cells were harvested from newborn and adult (3, 10, 20, 30, and 100 days) mice by using a staining medium (RPMI medium 1640 + 3% newborn calf serum)-filled syringe and resuspended at 25 × 106 cells/ml. Before usage, the medium was equilibrated at physiologic oxygen by keeping the medium for 24 h in the ≈5%O2 incubator.
FACS Analysis.
PerC suspensions from each mouse strain were preincubated with anti-CD16/CD32 mAb to block FcγRII/III receptors and stained on ice for 15 min with the following fluorochrome-conjugated antibodies in an 11-color staining combination: FITC-IgMa (DS-1) or FITC-IgD(11–26); PE-CD11b (M1/70) or PE-CD23 (B3B4); PECy5-CD5 (53–7.3); PECy5.5-CD21 (7G6) or PECy5.5-CD23 (B3B4); PECy7-IgD(11–26) or Cy7PE-CD11b (M1/70); Alexa594-IgMb (AF6–78.25); APC-B220 (RA3–6B2) or APC-IgMb (AF6–78.25); APCCy5.5-total IgM (331); APCCy7-total IgM (331); CasBlu-F4/80 and Qdot605-VH11. The fluorochrome-conjugated antibodies were either purchased from BD Pharmingen or custom-conjugated in our laboratory.
After washing away excess reagents, the stained cells were resuspended in staining medium containing 10 μg/ml propidium iodide (PI). Cells that stained with PI (dead cells) were excluded from analysis and/or sorting in all cases. Cells were analyzed and sorted on the Stanford shared FACS facility Hi-D FACS instruments, either on the FACSAria (Becton Dickinson) or a Stanford-built hybrid instrument (Flasher II) in which a FACS II bench is coupled to FACS DiVa electronics. Staining protocols were designed with CytoGenie software (ScienceXperts), and data were analyzed with FlowJo software (TreeStar).
To distinguish autofluorescent cells from cells expressing low levels of individual surface markers, we used FMO controls (17) in which cells were stained for all determinants except the one whose expression was being measured. During analysis, the FMO control was gated exactly like the fully stained sample to identify the target population on which the expression of the determinant was to be measured. This provided an upper threshold with which to distinguish autofluorescent cells from those expressing a determinant that binds the fluorescent reagent used to detect it (see Fig. 1).
FACS Sorting and Cell Transfer.
Thresholds were set for CD11b expression and other markers according to FMO control values. The sorting strategy adopted here (for cell transfer studies) was designed to add minimal amount of reagents (antibodies) on the B-1 cell surface to minimize unwanted cell activation. To enrich for B-1, live lymphocytes (FSclow, SSclow, PI−) were gated to first exclude CD3a+ (T cells) and CD23+ (B-2 cells) cells. The remaining cells were gated for sorting CD11b− and CD11b+ cells. B-1 purity among each sorted population constitutes ≈90% as determined by a parallel full-stained population and reanalysis after sort. PBS buffer containing 0.5 × 106 cells of each sorted population (CD11b− or CD11b+ cells) were injected i.p. into BAB/25 or RAG1-deficient mice. After 10–12 days, total PerC cells from injected mice were collected, stained, and analyzed as described.
Confocal Microscopy.
Total PerC from adult (BALB/c × CB.17)F1 mice were first stained by using a combination of reagents to include IgMa (FITC) and IgMb (Texas red) to be detected by the lasers set on the confocal microscopy. Live large B-1 were recognized by a gating strategy (PI−, FSchigh, IgMhigh, and CD11b+) and then sorted directly onto a coverslip contained in a 24-well plate. After sort, cells (in the coverslip) were precipitated, fixed in 4% paraformaldehyde for 15 min, washed in PBS, and air-dried. Finally, the coverslip was mounted by using a solution containing DAPI. The images were collected on a microscope (model Optiphot-2; Nikon) attached to a confocal laser scanning (model MRC1024; Bio-Rad) by using LaserSharp software (Bio-Rad). The laser lines on the krypton/argon laser were 488 nm (FITC), 568 nm (Texas red), and 647 nm (DAPI). Volocity 3.0 software (Improvision) was used to format and then merge the green (FITC), red (Texas red), and blue (DAPI) images.
Acknowledgments.
We thank Stanley Falkow (Stanford University) and Gregory Govoni, a fellow in Dr. Falkow's laboratory, for help in the preparation and analysis of the B-1 doublets by confocal microscopy and allowing us to use the confocal microscope Dr. Falkow's group maintains; Dr. Takeshi Fukuhara for discussion and help on the B-1 doublet project; Megan Philips and Ms. Ometa Herman for technical assistance; and John Mantovani for help in the preparation of this manuscript. E.E.B.G. thanks Dr. Sandro Rogerio de Almeida for encouragement, continuous support, and advice and Drs. Mario Mariano and Momtchilo Russo for the introduction to the Herzenbergs and support. This work was supported in part by National Institutes of Health Grant AI 076434. E.E.B.G. was supported by a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Division of the Brazilian Ministry of Education).
Footnotes
The authors declare no conflict of interest.
References
- 1.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann NY Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 2.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system: A model for the ontogeny and development of multiple lymphocyte lineages. Ann NY Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakawa K, et al. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 6.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Adoptive transfer of murine B-cell lineages. Ann NY Acad Sci. 1992;651:168–169. doi: 10.1111/j.1749-6632.1992.tb24610.x. [DOI] [PubMed] [Google Scholar]
- 7.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Alugupalli KR, Gerstein RM. Divide and conquer: Division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Knoops L, Louahed J, Renauld JC. IL-9-induced expansion of B-1b cells restores numbers but not function of B-1 lymphocytes in xid mice. J Immunol. 2004;172:6101–6106. doi: 10.4049/jimmunol.172.10.6101. [DOI] [PubMed] [Google Scholar]
- 10.Pers JO, Jamin C, Youinou P, Charreire J. Role of IL-10 in the distribution of B cell subsets in the mouse B-1 cell population. Eur Cytokine Netw. 2003;14:178–185. [PubMed] [Google Scholar]
- 11.Sen G, et al. Defective CD19-dependent signaling in B-1a and B-1b B lymphocyte subpopulations. Mol Immunol. 2002;39:57–68. doi: 10.1016/s0161-5890(02)00047-0. [DOI] [PubMed] [Google Scholar]
- 12.Tornberg UC, Holmberg D. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J. 1995;14:1680–1689. doi: 10.1002/j.1460-2075.1995.tb07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells SM, Kantor AB, Stall AM. CD43 (S7) expression identifies peripheral B cell subsets. J Immunol. 1994;153:5503–5515. [PubMed] [Google Scholar]
- 14.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastings WD, Gurdak SM, Tumang JR, Rothstein TL. CD5+/Mac-1- peritoneal B cells: A novel B cell subset that exhibits characteristics of B-1 cells. Immunol Lett. 2006;105:90–96. doi: 10.1016/j.imlet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: A guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 17.Roederer M. Spectral compensation for flow cytometry: Visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Curr Opin Immunol. 2007;19:150–155. doi: 10.1016/j.coi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 20.Herzenberg LA, Tung JW. B cell lineages: Documented at last! Nat Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 21.Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Andrade L, et al. Biased VH gene expression in murine CD5 B cells results from age-dependent cellular selection. Eur J Immunol. 1991;21:2017–2023. doi: 10.1002/eji.1830210908. [DOI] [PubMed] [Google Scholar]
- 23.Huetz F, Poncet P. Immunocompetence of peritoneal B cells. Res Immunol. 1989;140:75–87. doi: 10.1016/0923-2494(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 24.Huetz F, Sciard-Larsson EL, Pereira P, Portnoi D, Coutinho A. T cell dependence of the “natural” autoreactive B cell activation in the spleen of normal mice. Eur J Immunol. 1988;18:1615–1622. doi: 10.1002/eji.1830181022. [DOI] [PubMed] [Google Scholar]
- 25.Poncet P, Huetz F, Marcos MA, Andrade L. All VH11 genes expressed in peritoneal lymphocytes encode anti-bromelain-treated mouse red blood cell autoantibodies but other VH gene families contribute to this specificity. Eur J Immunol. 1990;20:1583–1589. doi: 10.1002/eji.1830200726. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]