Fig. 3.
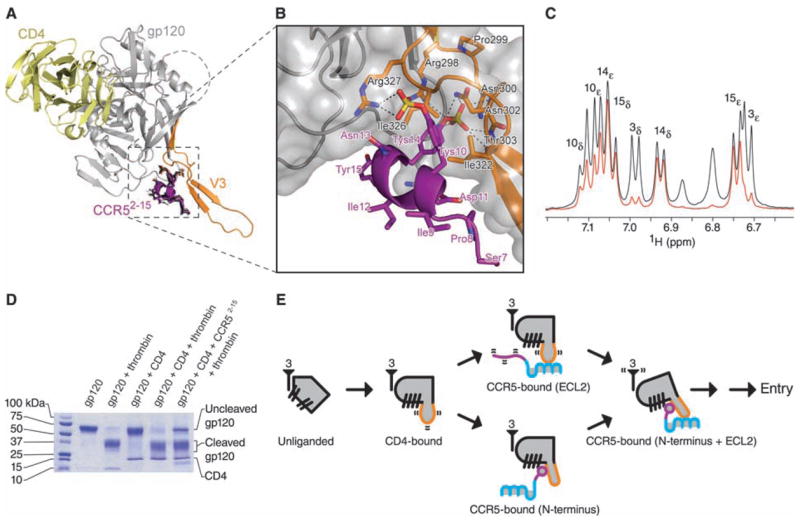
Interaction of the N terminus of CCR5 with HIV-1 gp120-CD4. (A) Molecular docking. The 20 lowest energy structures (black) from 200 docking runs of CCR52-15 are shown in stick representation. Despite initial random orientations, all favorable docking solutions had Tys 14 binding at the bridging sheet-V3 interface; none had Tys 10 at this cleft. Ribbon representations illustrate CD4 in yellow, gp120 in gray (with V3 in orange), and the lowest energy structure of CCR57-15 in purple. (B) Close-up, with molecular surface of gp120 in gray and select residues of gp120 (black labels) and CCR5 (purple labels) in stick representation. (C) Saturation transfer difference NMR spectrum of CCR52-15 in the presence of gp120–CD4 (red) overlaid on a control 1H spectrum (black). Experimental conditions were identical to those used for NOE experiments, except that the carrier was set at –1 and 50 parts per million for on- and off-resonance saturation, respectively. The intensities of the most strongly enhanced peaks (Tys 14 and Tyr 15) have been normalized to the corresponding signals in the control spectrum. Peak assignments made by 2D NMR (table S1) appear above their corresponding doublet signals. Tys 14 and Tyr 15 show strong saturation transfer difference effects, whereas Tys 10 shows a medium effect and Tyr 3 a very weak effect. These effects correlate directly with the buried surface area of each tyrosine ring in the docked structure. See fig. S9 for overlaid spectra employing 1 to 7 s saturation. (D) Effect of CCR52-15 on the proteolytic sensitivity of the V3. Electrophoresis on an 8 to 25% gradient SDS polyacrylamide gel shows the results of thrombin digestion on gp120 (core with V3; YU2 R5 strain of HIV-1) alone, or in the presence of sCD4 or sCD4 and CCR52-15 (35). (E) Structural intermediates of HIV-1 entry. At far left, a single monomer of unliganded gp120 (gray) is shown with separated β-hairpins. The threefold axis, from which gp41 interacts in the functional oligomer, is labeled with the number 3. In the CD4-bound state, the bridging sheet assembles, and the V3 (orange) is exposed and flexible. The next state involves either (upper pathway) the interaction of the CCR5-ECL2 region with the V3 tip or (lower pathway) the interaction of the CCR5 N terminus, which induces rigidification of the V3 stem. Engagement of CCR5 at both N terminus and ECL2 region triggers additional conformational changes leading to HIV-1 entry.
